Ignacio Oscar Procikieviez, Álvaro Alcaraz, Santiago Reimondez, Enzo Giordano Segade, Franco Signorini, Martin Maraschio, Lucio Ricardo Obeide
General Surgery Service, Hospital Privado Universitario de Córdoba. University Institute of Biomedical Sciences of Córdoba. Córdoba, Argentina.
Acta Gastroenterol Latinoam 2019;49(4):336-340
Recibido: 11/07/2018 / Aprobado: 12/05/2019 / Publicado en www.actagastro.org el 17/12/2019
Summary
A cephalic pancreaticoduodenectomy is the only option for patients with periampullary tumours. In some cases, the relation of the head of the pancreas with neighbouring vascular structures (superior mesenteric vein, portal vein or their confluence), may be the cause, in some occasions, of carrying out a vascular resection in the same surgical procedure, due to tumour involvement, to achieve an R0 resection. Multiple options for vein reconstruction have been described, considering the extension, the length, and the location of vein resection. Material and methods. A retrospective analysis was carried out on patients undergoing a cephalic pancreaticoduodenectomy with concomitant vascular resection and venous reconstruction with cryopreserved venous allograft. Their clinical outcomes as well as their morbidity and mortality were analysed. The patency of the graft was evaluated with ultrasound and computed tomography. Results. We present 5 patients who underwent venous (portal vein/superior mesenteric vein) resection and reconstruction with cryopreserved vein allografts. The average surgical time was 430 minutes. The anatomopathological diagnoses were 2 pancreatic ductal adenocarcinomas, 2 cholangiocarcinomas and a neuroendocrine tumour. Conclusion. Our experience in vein reconstruction with cryopreserved vein allografts shows that it is a safe and viable technique to be conducted in institutions with experience in hepato-pancreatic-biliary surgery and organ transplant.
Key words. Allograft, cryopreservation, pancreatic cancer, pancreaticoduodenectomy, portal vein.
Reconstrucción venosa con aloinjerto venoso criopreservado en duodenopancreatectomía. Cirugía pancreática
Resumen
La duodenopancreatectomía cefálica (DPC) es la única opción de cura en pacientes con tumores del área periampular. La relación de la cabeza del páncreas con estructuras vasculares vecinas (vena mesentérica superior, vena porta o su confluencia), conlleva, en algunas ocasiones, a realizar una resección vascular en el mismo acto quirúrgico, por compromiso tumoral, y así lograr una resección quirúrgica R0. Múltiples opciones para reconstrucción venosa han sido descriptas teniendo en cuenta la extensión, la longitud y el sitio de resección venosa. Material y métodos. Se analizaron retrospectivamente los pacientes sometidos a DPC con resección vascular concomitante y reconstrucción venosa con aloinjerto venoso criopreservado. Se analizaron los resultados postoperatorios, la morbilidad y la mortalidad. La permeabilidad del injerto fue evaluada con ultrasonido y tomografía computada. Resultados. Se presentan 5 pacientes a los cuales se les realizó resección venosa (vena porta/ vena mesentérica superior) y reconstrucción con aloinjerto venoso criopreservado. El tiempo quirúrgico promedio fue de 430 minutos. Los diagnósticos anatomopatológicos fueron 2 adenocarcinomas ductales de páncreas, 2 colangiocarcinomas y un tumor neuroendocrino. Conclusión. Nuestra experiencia en reconstrucción venosa con aloinjerto venoso criopreservado expone que se trata de una técnica segura y factible de realizar en centros con experiencia en cirugía hepatobiliopancreática y trasplante de órganos.
Palabras claves. Alograft, criopreservación, cáncer de páncreas, duodenopancreatectomía cefálica, vena porta.
The cephalic pancreaticoduodenectomy (CPD) is the only curative option in patients with pancreatic head tumours and in the periampullary area.1 As pancreatic resections have become surgically safer, indications for pancreatectomies have increased, and surgical techniques have evolved. A greater number of indications for intraductal papillary mucinous neoplasms (IPMN) and other cystic neoplasms have been described, as well as for chronic pancreatitis and metastasis of the head of the pancreas.2
Currently, although surgical resection increases survival in patients with localized disease, only between 10 and 20% of patients may be operated on at the time of diagnosis.3
The relationship of the head of the pancreas with neighbouring vascular structures (superior mesenteric vein, portal vein or its confluence); leads, on some occasions, to the need to perform a vascular resection in the same surgical act, due to tumour compromise, and thus achieve a surgical resection R0.4 Multiple options for vascular reconstruction have been described, considering the extension, length and site of the venous resection.
Material and methods
From January 2012 to October 2017, a total of 93 CPD were performed in the Hospital Privado Universitario de Córdoba. A retrospective analysis was carried out on patients undergoing concomitant vascular resection and venous reconstruction with cryopreserved venous allograft.
All the patients were studied preoperatively with a physical examination, laboratory tests, three-phase computed tomography (CT) of abdomen and/or abdominal magnetic resonance, as well as computed tomography of the thorax for correct staging. Each case was discussed in an interdisciplinary oncology committee composed of specialists in surgery, radiology, oncology and gastroenterology, thus evaluating the vein-tumour interface in a detailed manner.
Preoperative biliary drainage (endoscopic or percutaneous), was not carried out systematically and only performed in those patients with doubts about the final management or in symptomatic patients with a projected surgery delay of more than fifteen days. The definition of the International Study Group for Pancreatic Fistula (ISGPF) was adopted for postoperative pancreatic fistula (PPF) and delayed gastric emptying (DGE).5, 6 Complications were analyzed according to the Clavien-Dindo classification, considering as major complications grades III a / b and IV a / b.7
Operative mortality was defined as, death occurring within 30 days of the surgery, or during hospital admission if it was more than 30 days. The patency of the vascular anastomosis was analysed initially with a Doppler ultrasound within 24 hours of the postoperative period. It was later evaluated twice with a CT, a month and three months after surgery.
All patients signed written informed consent for the surgical procedure.
Surgical technique
The procedures that were carried out were CPD with pyloric preservation and standard lymphadenectomy; or Whipple procedure for cases in which, tumor involvement of the duodenum and/or pylorus was observed. The venous compromise was analyzed intraoperatively, and tangential or segmental resection was decided, based on the location and longitudinal and circumferential extension of the tumor lesion. Dissection and clamping were performed both, proximally and distally to the venous tumor compromise, to reduce potential bleeding during resection and reconstruction. The clamping time was monitored in all cases. When the venous resection was tangential without the possibility of performing a venorraphy due to venous caliber involvement, a cryopreserved venous graft patch was used. On the other hand, when the venous resection was segmental, the primary anastomosis of both ends was prioritized, and if not possible, a cryopreserved venous graft was interposed.
Graft procurement
The venous allografts were obtained during liver harvesting procedures performed by the liver and renal pancreatic transplant service in our hospital. Both iliac veins were obtained in each procedure, which were preserved in the University of Wisconsin solution at 4º for a maximum of 15 days. The ABO compatibility of the graft was respected in all patients.
Management and Postoperative Care
All patients underwent the immediate postoperative period in the Intensive Care Unit. Prophylaxis of deep vein thrombosis with low molecular weight heparin was started 24 hours postoperatively, depending on the Caprini score and the use of an epidural analgesia catheter by the anaesthesiology service, and antiaggregation was performed with 100 mg of acetylsalicylic acid every 24 hours from day 7 postoperatively, if there was no contraindication.
Results
Portal vein (PV), superior mesenteric vein (SMV), or portomesenteric confluent (PMC) resection was performed in 17 (18%) of the 93 patients analyzed, due to intraoperative suspicion of tumor venous infiltration.
Of the latter 17 patients, 11 (63%) were reconstructed with primary lateral venorrhapy or end to end anastomosis, 3 (18%) with politetrafluoroethylene (PTFE) and 5 (29%) received reconstruction with cryopreserved venous allograft.
Concerning about the cryopreserved venous allograft cases, 60% were men. The mean age of the patients undergoing surgery was 68 years (range: 56-75). The preoperative biliary drainage was performed in 1 case and no neoadjuvant chemotherapy was performed in any of them. The average surgical time was 430 minutes (range: 326-556) and 3 (60%) patients received transfusions during surgery.
Regarding venous resection, in 3 cases SMV resection was performed, 1 patient underwent PV resection and another at PMC resection (Figure 1). The average venous clamping time was 30 minutes (range: 25-40). In all cases the splenic vein was preserved, and in 1 patient the vein was reimplanted, because the resection compromised the union with the SMV.
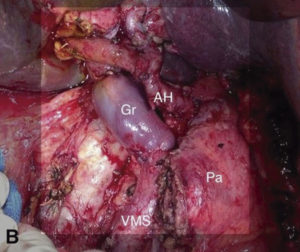
Figure 1. A) Intraoperative image after resection, where portal vein replacement is observed. Gr: venous graft. Pa: body and tail of pancreas. VMS: superior mesenteric vein. AH: Hepatic Artery. B) Ductal adenocarcinoma. Resection and replacement of superior mesenteric vein. Gr: venous graft. Pa: body and tail of pancreas. VMS: superior mesenteric vein.
The average time of hospitalization was 20 days (range: 13-30). Regarding morbidity, 40% (2 cases) presented major complications, PPF grade B in one patient and hospital-acquired pneumonia in the other. 2 patients (40%) presented DGE, which was resolved with medical treatment.
A single patient presented thrombosis of the anastomosis, performing re-laparotomy, thrombectomy and systemic heparinization, but he evolved into multiorgan failure and died on day 12 of the postoperative period. Early patency using Doppler and late patency one and three months after the surgery showed no compromise in the remaining 4 (80%) patients.
The venous segment was resected together with the surgical piece and sent to pathological anatomy. The anatomopathological diagnoses were 2 (40%) pancreatic ductal adenocarcinomas (pT3N1, pT3N0), 2 (40%) distal cholangiocarcinomas (T3N1, T2N0) and 1 (20%) neuroendocrine tumour (T3N0) (Figure 2). Vein wall infiltration was observed in 4 (80%) of the patients. The 5 patients (100%) showed R0 resections.
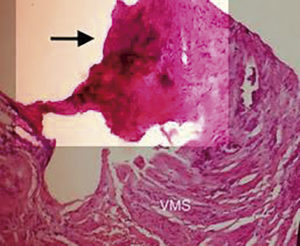
Figure 2. Microscopy image in which, the wall of the superior mesenteric vein is observed, its infiltration by ductal adenocarcinoma (100X). VMS: superior mesenteric vein. Black arrow: venous infiltration due to ductal adenocarcinoma.
Discussion
The invasion of neighboring vascular structures associated with pancreatic tumours is a problem that is frequently presented to the surgeon. Isolated tumoral infiltration of the porto-mesenteric venous axis is not an absolute contraindication for tumour resection with curative intent.8
Numerous retrospective studies affirm that, survival in patients undergoing CPD with venous resection compared to those without it, did not turn out to be statistically significant, as well as the anatomopathological result of a R1 vs. R0 resection. However, CPD with venous resection has shown to increase resectability rates in pancreatic tumour pathology. On the other hand, advances in imaging techniques and postoperative care have shown a decrease in morbidity and mortality associated with venous resection, even matching those reported in surgeries without vascular resections.3, 9, 10
Various types of grafts have been described and used for venous reconstruction after portomesenteric resections, always taking into account the location and extent of tumour vascular involvement.11, 12 In the case of segmental or tangential resections of short length, it is preferable to perform a primary end-to-end anastomosis or venorrhaphy, respectively, respecting the caliber and avoiding significant stenosis of the vein. Autologous vein grafts, such as the internal jugular vein, the inferior mesenteric vein, the superficial femoral vein, and the left renal vein; they proved to be a valid option, but they were associated with an increase in surgical time, the need for a second incision and complications at the graft extraction site.11, 12 Other option is synthetic materials, such as polytetrafluoroethylene (PTFE), but they have not shown benefits with respect to autologous grafts.13 Finally, the cryopreserved allografts have been used for venous reconstruction in various types of surgeries, starting with cadaveric donor liver transplantation and continuing with the contribution of Shi et al., who described the use of this type of grafts to reconstruct the middle suprahepatic vein in the right liver during living donor liver transplantation.14 Then, in 2012, Meniconi et al.,2 was the first to describe the use of cryopreserved venous allografts for venous reconstruction in CPD, reporting satisfactory results similar to those observed in our series with this technique. The benefits of allografts compared to other types of grafts are multiple. First of all, edema distal to venous extraction is avoided, as in the case of the autologous grafts; the risk of a second surgical site of infection is diminished, as well as the surgical time.15
In conclusion, we believe that such experience in venous reconstruction with cryopreserved venous allograft represents a feasible technique to perform in selected cases, in centres with experience in Hepatobiliopancreatic surgery and organ transplantation.
Conflict of interest. None declared.
Referencias
- Andolfi C, Bonavina L, Kavitt RT, Konda VJ, Asti E, Patti MG. Importance of Esophageal Manometry and pH Monitoring in the Evaluation of Patients with Refractory Gastroesophageal Reflux Disease: A Multicenter Study. J Laparoendosc Adv Surg Tech A 2016; 26: 548-550.
- Herbella FA, Andolfi C, Vigneswaran Y, Patti MG, Pinna BR. Importance of esophageal manometry and pH monitoring for the evaluation of otorhinolaryngologic (ENT) manifestations of GERD. A multicenter study. J Gastrointest Surg 2016; 20: 1673-1678.
- Andolfi C, Vigneswaran Y, Kavitt RT, Herbella FA, Patti MG. Laparoscopic Antireflux Surgery: Importance of Patient’s Selection and Preoperative Workup. J Laparoendosc Adv Surg Tech A 2017; 27: 101-105.
- Kleiman DA, Beninato T, Bosworth BP, Brunaud L, Ciecierega T, Crawford CV Jr, Turner BG, Fahey TJ 3rd, Zarnegar R. Early referral for esophageal pH monitoring is more cost-effective than prolonged empiric trials of proton-pump inhibitors for suspected gastroesophageal reflux disease. J Gastrointest Surg 2014; 18: 26-33.
- Patti MG, Arcerito M, Tamburini A, Diener U, Feo CV, Safadi B, Fisichella P, Way LW. Effect of laparoscopic fundoplication on gastroesophageal reflux disease-induced respiratory symptoms. J Gastrointest Surg 2000; 4: 143-149.
- Bello B, Zoccali M, Gullo R, Allaix ME, Herbella FA, Gasparaitis A, Patti MG. Gastroesophageal reflux disease and antireflux surgery-what is the proper preoperative work-up? J Gastrointest Surg 2013; 17: 14-20.
- Hom C, Vaezi MF. Extra-esophageal manifestations of gastroesophageal reflux disease: diagnosis and treatment. Drugs 2013; 73: 1281-1295.
- Roman S, Keefer L, Imam H, Korrapati P, Mogni B, Eident K, Friesen L, Kahrilas PJ, Martinovich Z, Pandolfino JE. Majority of symptoms in esophageal reflux PPI non-responders are not related to reflux. Neurogastroenterol Motil 2015; 27: 1667-1674.
- Hsu CS, Wen SH, Hung JS, Liu TT, Yi CH, Lei WY, Pace F, Chen CL. Overlap of Dyspepsia in Patients with Gastroesophageal Reflux Disease: Impact of Clinical, Metabolic, and Psychosocial Characteristics. Dig Dis Sci 2017; 62: 994-1001.
- Revicki DA, Wood M, Maton PN, Sorensen S. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med 1998; 104: 252-258.
- Chin KF, Myers JC, Jamieson GG, Devitt PG. Symptoms experienced during 24h pH monitoring and their relationship to outcome after laparoscopic total fundoplication. Dis Esophagus 2008; 21: 445-451.
- Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Functional Esophageal Disorders. Gastroenterology 2016; 150: 1368-1379.
- Neto SC, Herbella FA, Silva LC, Patti MG. Ratio between proximal/distal gastroesophageal reflux does not discriminate abnormal proximal reflux. World J Surg 2014; 38: 890-896.
- Oh JH, Kim TS, Choi MG, Lee H, Jeon EJ, Choi SW, Lee C, Chung IS. Relationship between Psychological Factors and Quality of Life in Subtypes of Gastroesophageal Reflux Disease. Gut Liver 2009; 3: 259-265.
- Jansson C, Nordenstedt H, Wallander MA, Johansson S, Johnsen R, Hveem K, Lagergren J. Severe gastro-oesophageal reflux symptoms in relation to anxiety, depression and coping in a population-based study. Aliment Pharmacol Ther 2007; 26: 683-691.
- Kessing BF, Bredenoord AJ, Saleh CM, Smout AJ. Effects of anxiety and depression in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2015; 13: 1089-1095.
Correspondencia: Ignacio Oscar Procikieviez
Naciones Unidas 346 (C.P.: 5000), Hospital Privado Universitario de Córdoba. Córdoba Capital, Argentina
Tel.: +54 0351 4688200
Correo electrónico: iproci8@hotmail.com
Acta Gastroenterol Latinoam 2019;49(4):336-340
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE