Esteban González1 ID· Diego Cordova Reyes2 ID· Paula Abad2 ID· Camila González2 ID· Juan José Cordero2 ID· Eduardo González3 ID· Guillermo López Dominguez2 ID· Juan Pesantez2 ID · Diego Tobar Lima2 ID
1 Departamento de Gastroenterología, Hepatología y Trasplante Hepático, Hospital Santa Inés. Departamento de gastroenterología. Universidad de Cuenca.
Cuenca, Ecuador.
2 Facultad de medicina. Universidad de Cuenca. Cuenca, Ecuador.
3 Departamento de Endoscopia del Hospital das Clinicas de la Facultad de Medicina de la Universidad de Sao Paulo, Brasil.
Acta Gastroenterol Latinoam 2024;54(1):70-78
Received: 25/12/2023 / Accepted: 20/02/2024 / Published online: 25/03/2024 /
https://doi.org/ 10.52787/agl.v54i1.381
Summary
Introduction. Helicobacter pylori is responsible for a highly prevalent bacterial infection in developing countries, and its adequate pharmacological treatment is essential to prevent serious complications such as gastric cancer. However, at present, the usual treatment has significant failure rates, and its main factor is bacterial resistance. Aim. To identify the frequency and risk factors for resistance to clarithromycin, amoxicillin, azithromycin, metronidazole, and levofloxacin in patients with Helicobacter pylori infection in the Gastroenterology Center, Cuenca, between December 2020 and May 2021. Material and methods. A single-center observational and descriptive study of 157 adult patients who underwent endoscopic procedures and biopsies that were cultured to obtain resistance rates to different antibiotics. Patients were excluded if they did not sign the informed consent if they had received treatment with antibiotics, bismuth and/or proton pump inhibitors, at least two weeks before the procedure, and if they had received prior treatment for Helicobacter pylori, or with upper gastrointestinal bleeding. Information was collected using a form: age, sex, sociodemographics, and access to public services. Data were presented in tables, with relative frequencies and percentages. Pearson’s Chi-square test was used to assess the association between variables. Statistically significant association when p < 0.05. Results. The prevalence of H. pylori infection was 31.2% by urea test and 12.1% by 48-hour culture. The lowest percentage of resistance was observed for levofloxacin (0%), followed by azithromycin at 16.7%; clarithromycin at 21.1%; amoxicillin 25 mcg at 26.3%; amoxicillin 10 mcg at 31.6% and with the highest percentage of resistance, metronidazole at 63.2%. Resistance to metronidazole and amoxicillin 25 mcg was only significantly associated with patient age, being higher in those older than 60 years.
Conclusions. Metronidazole had a high resistance rate in the sample, while levofloxacin had a sensitivity of 100%. Resistance to metronidazole and amoxicillin 25 mcg was associated with patient longevity.
Keywords. Helicobacter pylori, antibiotic resistance, antibiotic scheme, antibiogram.
Prevalencia y factores de riesgo asociados a la resistencia antibiótica al Helicobacter pylori en Cuenca, Ecuador
Resumen
Introducción. Helicobacter pylori es responsable de una infección bacteriana de alta prevalencia en los países en vías de desarrollo y su tratamiento farmacológico adecuado es esencial para prevenir complicaciones graves como el cáncer gástrico. Sin embargo, en la actualidad, el tratamiento habitual presenta tasas importantes de fracaso, y se considera que el principal factor es la resistencia bacteriana. Objetivo. Identificar la frecuencia y factores de riesgo de resistencia a claritromicina, amoxicilina, azitromicina, metronidazol y levofloxacina en pacientes con infección por Helicobacter pylori en el Centro de Gastroenterología de Cuenca, entre diciembre de 2020 y mayo de 2021. Materiales y métodos. Estudio observacional y descriptivo unicéntrico que incluyó 157 pacientes adultos estudiados mediante procedimientos endoscópicos y biopsias que fueron cultivadas para obtener tasas de resistencia a diferentes antibióticos. Se excluyeron los pacientes que no firmaron el consentimiento informado, que habían recibido tratamiento con antibióticos, bismuto y/o inhibidores de la bomba de protones al menos dos semanas antes del procedimiento, y aquellos que habían recibido tratamiento previo para Helicobacter pylori, o presentaron hemorragia digestiva alta. Se recogió información mediante un formulario: edad, sexo, sociodemografía y acceso a servicios públicos. Los datos se presentaron en tablas, con frecuencias relativas y porcentajes. Se utilizó la prueba Chi-cuadrado de Pearson para evaluar la asociación entre variables. Se consideró asociación estadísticamente significativa cuando p < 0,05. Resultados. La prevalencia de la infección por Helicobacter pylori fue del 31,2% mediante la prueba de urea y del 12,1% por el cultivo de 48 horas. El menor porcentaje de resistencia se observó para levofloxacina (0%), seguido de azitromicina con 16,7%; claritromicina con 21,1%; amoxicilina 25 mcg con 26,3%; amoxicilina 10 mcg con 31,6% y, con el mayor porcentaje de resistencia, metronidazol con 63,2%. La resistencia a metronidazol y amoxicilina 25 mcg solo se asoció significativamente con la edad de los pacientes, siendo mayor en los mayores de 60 años. Conclusiones. El metronidazol presentó una elevada tasa de resistencia en la muestra, mientras que la levofloxacina tuvo una sensibilidad del 100%. La resistencia al metronidazol y a la amoxicilina se asoció con la longevidad de los pacientes.
Palabras claves. Helicobacter pylori, resistencia a antibióticos, esquema antibiótico, antibiograma.
Introduction
Helicobacter pylori (H. pylori) infection is a global health problem, with a high prevalence, ranging from 85 – 90% in developing countries to 30-50% in developed countries.1 However, its prevalence in each geographical area may differ. In Ecuador, it has been shown that it can vary between 40.2% – 47.66% in adults.2-4 It is a microaerophilic, gram-negative, helicoidal bacterium that is transmitted via fecal-oral, oral-oral, contaminated food and water. Its pathogenicity is related to its strong ability to survive in the acidity of the human stomach (pH below 4) through the activity of three enzymes: urease, oxidase, and catalase.5 The most important for its diagnostic utility is urease, an enzyme accumulated in the cytoplasm of the microorganism. This enzyme catabolizes urea into two components, ammonia, and carbon dioxide, thus increasing the pH (between 6 and 7) and forming an alkaline medium that allows it to neutralize the acid and survive. The essential protective layer of the stomach, which prevents acid aggression to the mucosa, is attached to the gastric mucus. Its pathogenicity also includes processes such as activation of the inflammatory response and evasion of the immune system.5 This microorganism is responsible for a wide spectrum of gastrointestinal pathologies, ranging from non-specific disorders such as dyspepsia to preneoplastic lesions. It is considered responsible for up to 90% of duodenal ulcers, a large proportion of atrophic gastritis, 92% of MALT-type lymphomas and increases the risk of gastric adenocarcinoma by a factor of six. For this reason, early treatment is important.6 There are several methods of diagnosis, but the choice of one or the other varies greatly depending on the geographical area. In the case of the urea breath test, it is a non-invasive method that, according to the recommendations of the Maastricht consensus, may be more cost-effective in countries with a high prevalence of infection.1 However, there are countries where the complications of this infection are more frequent, such as Ecuador, where the high incidence of gastric cancer justifies the use of invasive procedures such as upper gastrointestinal endoscopy with biopsy. Treatment is carried out with different antibiotic regimens, depending on the pattern of antibiotic resistance, adverse effects, or adherence, etc. The most used regimen in Ecuador is triple therapy consisting of amoxicillin, clarithromycin and proton pump inhibitors (PPIs ) with an effectiveness of approximately 80%.7, 8 It should be noted that antibiotic therapy should be initiated early as secondary prevention of complications of this infection. However, there is a significant rate of failure in eradicating H. pylori due to the development of bacterial resistance. In Quito, Ecuador, up to 32% of cases may be resistant to the amoxicillin/clarithromycin combination.8, 9 In the literature, H. pylori resistance rates show differences according to geographical area and vary for each antibiotic, with high resistance found mainly against amoxicillin and metronidazole.8-10 Antibiotic resistance is associated with educational, sociodemographic and cultural variables. However, there are no studies in our environment on the prevalence of bacterial resistance to antibiotic therapy determined with the gold standard technique, i.e. the use of cultures with susceptibility tests. The Maastricht VI consensus points out the importance of local or regional bacterial susceptibility studies to select the most appropriate antibiotic regimen, as there is wide geographical variability in antibiotic resistance rates and differences between individuals depending on their previous exposure and access to antibiotics.11
Aim
The present study aims to analyze the prevalence of antibiotic resistance of Helicobacter pylori strains and their associated risk factors in infected and endoscopically analyzed patients.
Materials and Methods
An observational, descriptive, and cross-sectional study was conducted, which included patients from a gastroenterology specialty center, located in the city of Cuenca, Ecuador. They were selected during the period between December 2020 and May 2021, through non-probabilistic purposive sampling, based on the application of the selection criteria. All patients who underwent upper gastrointestinal endoscopy with biopsy during this period, who were over 18 years of age and who signed the informed consent, were included. Patients were excluded if they did not sign the informed consent, if they had received antibiotics, bismuth and/or proton pump inhibitors at least two weeks prior to the procedure, if they had received prior treatment for H. pylori, if they had upper digestive bleeding, if there were problems with biopsy collection, and/or if the biopsy sample was insufficient.The endoscopic study performed allowed the collection of samples from the antrum and the gastric body according to the current Maastricht VI recommendations.11
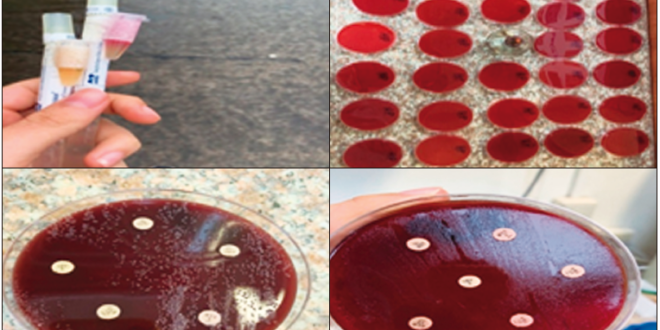
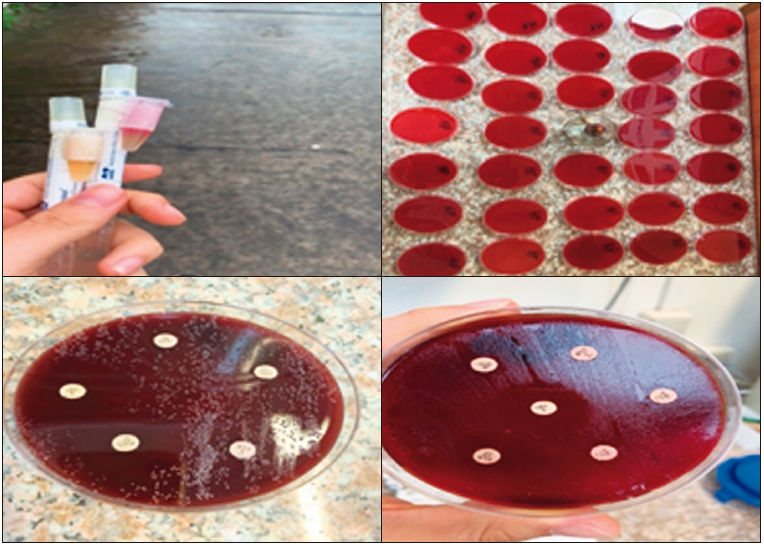
One biopsy was taken from the gastric antrum for the rapid urease test, while two biopsies (a single biopsy from the antrum and one from the body) were taken for culture, using the standardized method of preparing a culture medium based on Columbia blood agar (Oxoid) plus a Campylobacter Blaser-Wang supplement, using Stuard’s transport medium (bacterial viability in
48 hours), and taken to the laboratory for culture in less than 5 hours. The biopsy fragments were placed in a mortar with transport medium and enrichment broth until completely homogenized. The culture medium used was Pylori Agar in combination with horse plasma that facilitated the growth of bacteria with PolyVitex and antibiotics, which prevented the growth of enterobacteria, allowing only the development of H. pylori colonies. Once the medium was prepared, the sample was plated and incubated at 35ºC under microaerophilic conditions (low oxygen tension environment and 10-12% CO2) for a minimum of 3 days and a maximum of 5 days. Subsequently, streaking was used for colony counting and streak separation for bacterial identification by examining discrete translucent, on-coalescing colonies. A Gram stain study was performed to confirm the presence of H. pylori by visualizing of Gram-negative, curved, or spiral-shaped bacilli. Once the H. pylori culture was confirmed, an antibiogram was performed by Kirby Bauer technique, using antibiotic discs of clarithromycin (15 mcg), amoxicillin (10 mcg), amoxicillin (25 mcg), metronidazole (5 mcg), levofloxacin (15 mcg), azithromycin (15 mcg).
The cut-off points and standardization for the determination of sensitivity and resistance were applied according to the Clinical and Laboratory Standards Institute.12 The following antibiotics were defined as sensitive according to their halo diameter: amoxicillin ≥ 25 mm, clarithromycin ≥ 21 mm; metronidazole
≥ 21 mm, intermediate between 16 and 21 mm, and resistant if < 16 mm. As a cut-off point for levofloxacin, > 19 mm is sensitive and less than 17 mm is resistant. For azithromycin, a halo ≥ 22 mm was defined as sensitive and < 16 mm as resistant.9,13
In addition, a questionnaire was administered to each patient, in which general variables such as age, sex, residence and educational level were collected, as well as questions on whether they had access to drinking water, sewage and the frequency of deworming, justified by the fact that the Ministry of Public Health of Ecuador has a prevention program that uses metronidazole periodically to deworm the population in our environment, variables that indicate environmental conditions and unhealthy habits associated with the prevalence of H. pylori infection.
Statistical Analysis: Based on the stated objectives, data analysis was performed using SPSS statistical software version 23. First, the frequency of H. pylori infection and antibiotic resistance was determined. From this information, distribution tables were generated for all variables, as well as dispersion and location statistics according to each variable, with relative frequencies and percentages. Pearson’s Chi-square test was used to evaluate the association between variables. Statistically significant association when p < 0.05.
Ethical Considerations: This research complies with current medical research regulations and its development was authorized by the authorities of the Gastroenterology Center and the University of Cuenca Health Area Research Bioethics Committee (COBIAS). Informed consent was obtained from the patients for the use of the samples collected and the collection of socio-demographic information. The authors declare that this article does not contain any personal information that would allow the identification of participants.
Figure 1. Transport medium and culture with antibiogram
Results
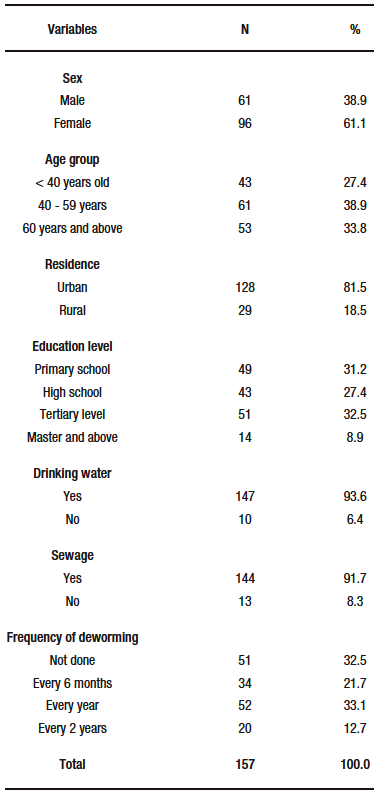
A total of 157 cultures with antibiograms were studied. Of the participants, 61.1% were female. The most frequent age group was 40-59 years with 38.9% (n=61) followed by the group ≥ 60 years with 33.8% (n=53). Residence, education, availability of drinking water sewage and frequency of deworming are summarized in Table 1.
Table 1. General characteristics of the study population
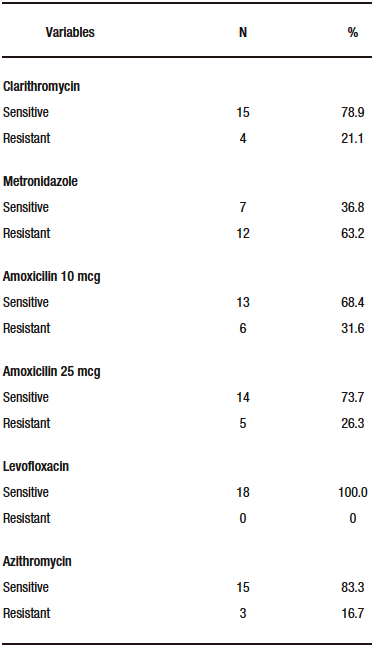
The prevalence of H. pylori infection was 31.2% (n=49) by urea test and 12.1% (n=19) by 48-hour culture. Resistance patterns identified by antibiogram
(Table 2) to H. pylori positive cultures showed high resistance to metronidazole with 63.2% and amoxicillin with 31.6%.
Table 2. Bacterial resistance patterns in cultures positive for H. pylori
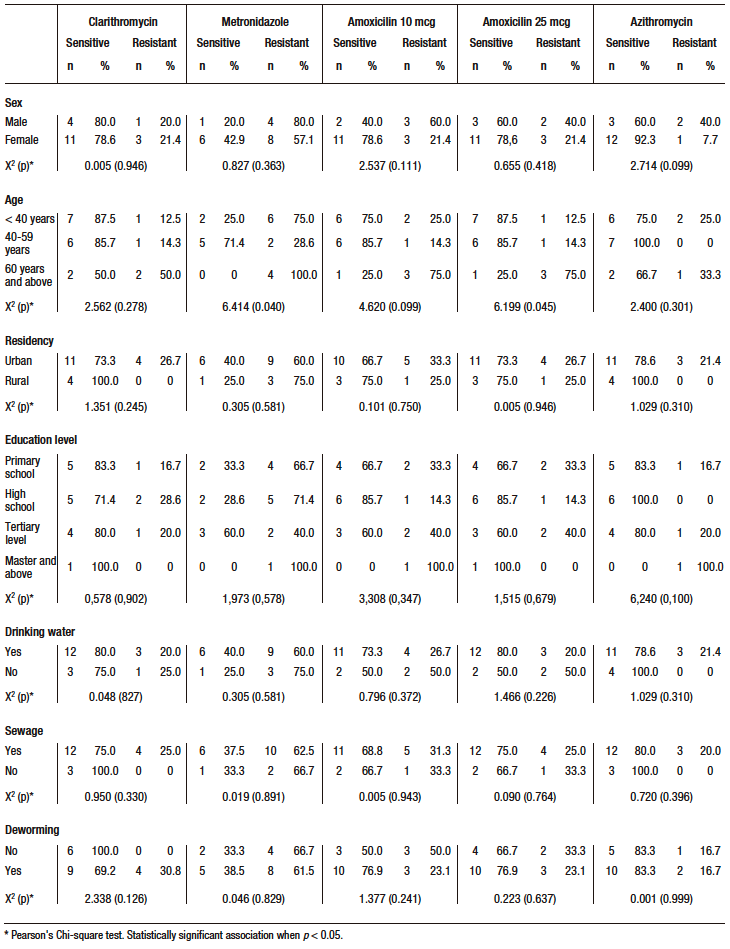
A statistically significant association was found between age and resistance to metronidazole (X2 = 6.414; p = 0.040) and amoxicillin 25 (X2 = 6.199; p = 0.045), with older subjects (≥ 60 years) having a higher prevalence of bacterial resistance (100% resistance to metronidazole and 75.0% resistance to amoxicillin). The rest of the variables did not show a statistically significant association with antimicrobial resistance (Table 3).
Table 3. Factors associated with bacterial resistance in H. pylori
Discussion
This is an observational study that analyzes the prevalence of H. pylori infection, antibiotic resistance, and factors associated with the latter in patients with an indication for endoscopic study, in order to identify these variables in our population. We provide the following novel information: the prevalence of H. pylori infection is 31.2% by urea test and 12.1% by 48-hour culture. The difference observed between the two prevalences is probably due to an overestimation by the rapid urease test. In this regard, this test has been questioned for its inability to rule out the presence of other urease-producing bacteria in the gastric mucosa.14 Evidence suggets that its sensitivity is low, particularly in situations where the density of H. pylori is low.14,15 Although the 48-hour culture presents better sensitivity and specificity indexes, this technique also has its disadvantages, such as sample processing time, transport medium, culture and/or incubation time. These factors could negatively influence its diagnostic capacity.15,16 However, they were taken into account in this study to avoid influencing the results.
In addition, this study demonstrated significant resistance to metronidazole in 63.2% of cases, amoxicillin in 31.6% and clarithromycin in 21.1%. This resistance is significantly associated with the older patient age. Antibiotic resistance due to H. pylori is frequent in Latin America; with an estimated 80% efficacy and 32% in vitro resistance to first-line amoxicillin/clarithromycin, according to a study conducted in Quito-Ecuador.7–9
In our study, we identified the highest antibiotic resistance of H. pylori against metronidazole in 63.2% of the cases. This correlates with high levels found in other studies in different geographical areas: Quito 63%, Colombia 81%, Brazil 38.9%, Cameroon 97.85%, China 78% and Portugal 33.9%.8, 9,13, 17, 18 This shows a high rate of resistance to metronidazole, in most cases higher than 50%. The differences are probably due to the study design and the sociodemographic characteristics of the populations studied. In our study, amoxicillin resistance was determined in 31.6% of the strains. Similar studies have shown great variability in resistance: Quito 43%, Colombia 3.8%, Portugal 0.6%, China 9%, Dominican Republic 26.76%, Cameroon 97% and Brazil 68.8%.8-10,13,17,18 The high variability could be explained by the pattern of use and previous exposure to B-lactams in each population studied, being one of the main antibiotics used since childhood for the treatment of multiple respiratory infections. In the case of resistance to clarithromycin, 21.1% of the strains were found to be resistant. This correlates with similar levels found in Bogota with 17.7%, Brazil with 23.2% and Germany with 28.2%.13,17,19 However, in other areas, it can vary significantly, as seen in Quito with 66%, in Ireland with 7.3%, and Malaysia with 0%.8,17 This is probably due to the pattern of previous use in each geographic area. Nevertheless, this resistance should be considered important since, according to the Maastricht VI consensus, the use of an antibiogram is recommended or its use should be avoided in local resistance rates greater than 15%.11
Our study highlights the fact that 100% sensitivity to levofloxacin was found, which correlates with the study from the Dominican Republic that found the same situation.10 However, in China and Portugal, they identified a resistance profile of 31% and 33.9% respectively.17,18 This difference may be due to the widespread use of fluoroquinolones in first world countries for multiple infections, which translates into access and previous exposure of the population. Regarding factors associated with bacterial resistance, age was the only factor significantly associated with metronidazole (p = 0.040) and amoxicillin (p = 0.045). Vallejos et al. also reported that metronidazole resistance was significantly higher in subjects older than 60 years, while clarithromycin did not show a defined resistance pattern across age groups.20 In previous studies, Almeida et al. and Alban et al. observed that being female was an independent predictor of antibiotic resistance, especially for clarithromycin and/or metronidazole.7, 18 Similarly, in the study by Osato et al. women and young adults harbored resistant H. pylori more frequently than men and older patients.21 In contrast, Wang et al. found a significantly higher rate of clarithromycin resistance in men than in women (44.4% vs. 15.2%, respectively; OR: 4.5 p = 0,002).17 Previous and failed eradication treatments were also associated with lower susceptibility to clarithromycin. In addition, a history of frequent infections, a first-degree relative with gastric cancer, and a low level of education were associated with greater resistance to levofloxacin.18 Another study by Gomollon et al.22 observed that among patients without previous H. pylori eradication treatment, there was a higher proportion of resistance to clarithromycin in those older than 40 years. These findings suggest that age could be a risk factor for antimicrobial resistance, because the older you get, the more often you use antibiotics.
Regarding the demographic area, as in our study, Osato et al. found no significant regional differences in antibiotic resistance.21 However, despite the documented risk factor represented by the consumption of untreated or non-drinking water, the absence of sewerage for wastewater, or the indiscriminate use of metronidazole as an antiparasitic agent, these variables did not have a significant association with antimicrobial resistance in this study.23, 24
Some of the limitations recognized in our study are that we did not use the dilution method preferred by the European Committee for Antimicrobial Susceptibility Testing (EUCAST), so the minimum inhibitory concentration (MIC) level was not analyzed, which makes comparison with other studies difficult. Another limitation is the difficulty in extrapolating the data to the population of Cuenca due to the limited number of samples analyzed, so it is recommended that this study be carried out with larger samples.
Conclusion
In conclusion, antibiotic resistance to H. pylori is a public health problem, because it is one of the most common gastroenterological infections worldwide. Proper selection of an antibiotic regimen as initial therapy avoids exposure to nonspecific antibiotics and increase the cure rate. A significant percentage of the therapies used are resistant, with metronidazole (63.2%) being the most resistant, followed by amoxicillin (31.6%), clarithromycin (21.1%), and azithromycin (16.7%). Levofloxacin was the only drug that was sensitive in 100% of cases. Age over 60 years was significantly associated with resistance to metronidazole and amoxicillin 25 mcg.
The antibiotic scheme with levofloxacin and azithromycin could be considered as the first treatment option in patients over 60 years of age in the city of Cuenca, Ecuador.
Consent for Publication. Written informed consent was obtained from the patient or their parent, guardian, or relative to publish the data and/or clinical images for the benefit of science. A copy of the consent form is available to the editors of this journal.
Intellectual Property. The authors declare that the data, figures and tables that appear in this article are original and were made in their belonging institutions.
Funding. The authors declare that there were no external sources of funding.
Conflict of interest. The authors declare that they have no conflicts of interest related to this article.
Copyright
© 2024 Acta Gastroenterológica latinoamericana. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license, which allows non-commercial use, distribution, and reproduction, provided the original author and source are acknowledged.
Cite this article as: González E, Cordova-Reyes D, Abad P et al. Prevalence and Risk Factors Associated with Helicobacter pylori Antibiotic Resistance in Cuenca, Ecuador. Acta Gastroenterol Latinoam. 2024;54(1):70-78. https://doi.org/ 10.52787/agl.v54i1.381
References
- Khoder G, Muhammad JS, Mahmoud I, Soliman SSM, Burucoa C. Prevalence of Helicobacter pylori and Its Associated Factors among Healthy Asymptomatic Residents in the United Arab Emirates. Pathogens. 2019;8(2):44.
- Buitrón V, Catalina P. Prevalencia por infección por Helicobacter pylori y asociación con patalogías gástricas en pacientes adultos de chequeo ejecutivo desde enero del 2010 hasta septiembre del 2012 del Hospital Metropolitano de Quito-Ecuador. [Internet] [bachelorThesis]. [Quito, Ecuador]: Universidad San Francisco de Quito; 2013 [citado el 10 de febrero de 2024]. Disponible en: http://repositorio.usfq.edu.ec/handle/23000/1503
- Aroca Albiño JM, Vélez Zamora L. Prevalencia de Helicobacter pylori en pacientes asintomáticos en Ecuador. revistavive. 2021;4(11):193-202.
- Icaza JDL, Cruz CPV. Prevalencia del Helicobacter pylori mediante antígeno en heces en pacientes sintomáticos del Centro Ambulatorio en Guayaquil-Ecuador. RECIMUNDO. 2019;3(4):78-92.
- Cervantes-García E. Helicobacter pylori: mecanismos de patogenicidad. Rev Mex Patol Clin Med Lab. 2016;63(2):100-9.
- Marinho JR, Averbach M, Deguti MM, Rodriguez TN. Tratado de Gastroenterologia: da Graduação à Pós-graduação. 2a edição. Zaterka S, Eisig JN, editores. São Paulo: Editora Atheneu; 2016. 1550 p.
- Alban O, Medina C, Urquiaga T. Incidencia de resistencia a tratamiento convencional de Helicobacter pylori, en una población adulta de Cajamarca. Revista Caxamarca. 2018;17:103-10.
- Reyes Chacón JA, Guzmán Guerrero KV, Pacheco Tigselema RE, Pazmiño Quirós GF, Morales Ñacato EJ, Escalante Vanoni LS. Susceptibilidad antibiótica de Helicobacter pylori: un estudio de prevalencia en pacientes con dispepsia en Quito-Ecuador. Rev Colomb Gastroenterol. 2017;32(4):305-10.
- Kouitcheu Mabeku LB, Eyoum Bille B, Tepap Zemnou C, Tali Nguefack LD, Leundji H. Broad spectrum resistance in Helicobacter pylori isolated from gastric biopsies of patients with dyspepsia in Cameroon and efflux-mediated multiresistance detection in MDR isolates. BMC Infect Dis. 2019;19(1):880.
- Camarena J, Khoury L. Hallazgos recientes de Helicobacter pylori resistente a antibióticos en la República Dominicana. Cienc Salud. 2019;3(3):25-33.
- Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;gutjnl-2022-327745.
- Clinical and Laboratory Standards Institute. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of In Frequently Isolated or Fastidious Bacteria. 3er edition. Wayne, PA: Clinical and Laboratory Standards Institute: CLSI guideline M45.;2016.19 p.
- Ogata SK, Gales AC, Kawakami E. Antimicrobial susceptibility testing for Helicobacter pylori isolates from Brazilian children and adolescents: comparing agar dilution, E-test, and disk diffusion. Braz J Microbiol. 2014;45(4):1439-48.
- Penny Z, Reynolds IS, Buckley M, Martin ST. Rapid urease testing in isolation is inadequate during invasive testing for H. pylori. Ir J Med Sci. 2022;191(2):963-4.
- Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G. Diagnosis of Helicobacter pylori: what should be the gold standard? World J Gastroenterol. 2014;20(36):12847-59.
- Jearth V, Rath MM, Chatterjee A, Kale A, Panigrahi MK. Drug-Resistant Helicobacter pylori: Diagnosis and Evidence-Based Approach. Diagnostics (Basel). 2023;13(18):2944.
- Wang D, Guo Q, Yuan Y, Gong Y. The antibiotic resistance of Helicobacter pylori to five antibiotics and influencing factors in an area of China with a high risk of gastric cancer. BMC Microbiol. 2019;19(1):152.
- Almeida N, Romãozinho JM, Donato MM, Luxo C, Cardoso O, Cipriano MA, et al. Helicobacter pylori antimicrobial resistance rates in the central region of Portugal. Clinical Microbiology and Infection. 2014;20(11):1127-33.
- Trespalacios AA, Otero Regino W, Mercado Reyes M. Resistencia de Helicobacter pylori a metronidazol, claritromicina y amoxicilina en pacientes colombianos. Revista colombiana de Gastroenterología. 2010;25(1):31-8.
- Vallejos M C, Garrido O L, Cáceres L D, Madrid AM, Defilippi C, Defilippi C C, et al. Prevalencia de la resistencia a metrodinazol, claritromicina y tetraciclina en Helicobacter pylori aislado de pacientes de la Región Metropolitana. Rev méd Chile. 2007;135(3):287-93.
- Osato MS, Reddy R, Reddy SG, Penland RL, Malaty HM, Graham DY. Pattern of Primary Resistance of Helicobacter pylori to Metronidazole or Clarithromycin in the United States. Arch Intern Med. 2001;161(9):1217.
- Gomollón F, Santolaria S, Sicilia B, Ferrero M, José Revillo M, Ducóns J, et al. Resistencia de Helicobacter pylori al metronidazol y a la claritromicina: análisis descriptivo entre 1997 y 2000. Medicina Clínica. 2004;123(13):481-5.
- Gnida A, Felis E, Ziembińska-Buczyńska A, Łuczkiewicz A, Surmacz-Górska J, Olańczuk-Neyman K. Evidence of mutations conferring resistance to clarithromycin in wastewater and activated sludge. 3 Biotech. 2020;10(1):7.
- H GZ, Zurita J, Oñate X, Espinosa Y. Patrones de resistencia secundaria de Helicobacter pylori a metronidazol y claritromicina en Quito. Rev Fac Cienc Médicas Quito. 2001;26(2-3):24-75-51.
Correspondence: Esteban González
Email: tebogonzalez@hotmail.com
Acta Gastroenterol Latinoam 2024;54(1):70-78
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE