Andrés Fernando Rodríguez-Gutiérrez1 ID· Camilo Andrés Duarte-Calderón2 ID· Sergio Mauricio Moreno López3 ID
1 Universidad Nacional de Colombia, Universidad de los Andes, Hospital Universitario Nacional de Colombia.
2 Universidad Nacional de Colombia.
3 Universidad de los Andes.
Colombia.
Acta Gastroenterol Latinoam 2024;54(1):42-55
Received: 03/02/2024 / Accepted: 15/03/2024 / Published online: 25/03/2024 /
https://doi.org/10.52787/agl.v54i1.390
Summary
Introduction. The liver is an organ that is affected by multiple mechanisms in the presence of COVID-19. The aim of this study was to determine whether elevated alanine aminotransferase, aspartate aminotransferase, and total bilirubin levels are predictors of mortality in adults with COVID-19. Materials and methods. A retrospective cohort study of adults hospitalized between 2020 and 2022 at a university hospital in Bogotá for COVID-19 and hypoxemia. All-cause mortality was the primary outcome. An independent multivariate model was built for each of the following markers of liver injury: alanine aminotransferase, aspartate aminotransferase, and total bilirubin. Each model was adjusted by age, presence of diabetes mellitus, presence of fever during hospitalization, lymphocyte count, D-dimer, and lactate dehydrogenase. Results. A total of 704 patients were included. The mortality rate was 38%. Elevated alanine aminotransferase, aspartate aminotransferase, and total bilirubin levels on admission were reported in 64%, 64%, and 8.3% of patients, respectively. According to the multivariate analysis, the elevation of both aspartate aminotransferase (OR = 1.06, 95% CI: 1.02-1.11 for each 40 U/L increase, p – value = 0.009) and total bilirubin levels (OR = 1.26, 95% CI: 1.08 -1.47 for every rise in 1mg/dl, p – value = 0.003) were independently associated with death. Total bilirubin level was also associated with intensive care unit admission, need for invasive mechanical ventilation and length of hospital stay. The results for alanine aminotransferase did not allow us to conclude an independent association with death. Age, fever, and lowest lymphocyte count during hospitalization were also associated with death. Conclusion. Elevated transaminases and total bilirubin levels are frequent findings in patients with COVID-19 and hypoxemia. Aspartate aminotransferase and total bilirrubin were predictive of mortality in these patients, so their measurement on admission is a reasonable practice. Progress needs to be made in incorporating these markers into predictive models of mortality and clinical decision rules.
Keywords. COVID-19, mortality, liver function tests, transaminases, bilirubin, prognosis.
Elevación de los niveles de transaminasas y bilirrubina total como marcadores pronósticos de mortalidad en adultos hospitalizados por COVID-19. Un estudio de cohorte
Resumen
Introducción. El hígado es un órgano que se ve afectado por múltiples mecanismos en presencia de COVID-19. El objetivo de este estudio fue determinar si los niveles elevados de alanina aminotransferasa, aspartato aminotransferasa y bilirrubina total son predictores de mortalidad en adultos con COVID-19. Materiales y métodos. Estudio de cohorte retrospectivo de adultos hospitalizados entre 2020 y 2022 en un hospital universitario de Bogotá por COVID-19 e hipoxemia. La mortalidad por todas las causas fue el resultado primario. Y se construyó un modelo multivariado independiente para cada uno de los siguientes marcadores de lesión hepática: alanina aminotransferasa, aspartato aminotransferasa y bilirrubina total. Cada modelo se ajustó por edad, presencia de diabetes mellitus, presencia de fiebre durante la hospitalización, recuento de linfocitos, dímero D y lactato deshidrogenasa. Resultados. Se incluyó a un total de 704 pacientes. La tasa de mortalidad fue del 38%. Los niveles elevados de alanina aminotransferasa, aspartato aminotransferasa y bilirrubina total al ingreso se registraron en el 64%, 64% y 8,3% de los pacientes, respectivamente. Según el análisis multivariado, la elevación de los niveles de aspartato aminotrasferasa (OR = 1.06; IC 95%: 1.02 – 1.11 por cada aumento de 40 U/L, valor de p = 0.009) y de bilirrubina total (OR = 1.26, IC 95%: 1.08 -1.47 por cada aumento de 1mg/dl, valor de p = 0.003) se asociaron de forma independiente con la muerte. El nivel de bilirrubina total también se asoció con el ingreso en la unidad de cuidados intensivos, la necesidad de ventilación mecánica invasiva y la duración de la estancia hospitalaria. Los resultados de la alanina aminotransferasa no permitieron concluir una asociación independiente con la muerte. La edad, la fiebre y el recuento más bajo de linfocitos durante la hospitalización también se asociaron con la muerte. Conclusión. Los niveles elevados de transaminasas y bilirrubina total son hallazgos frecuentes en pacientes con COVID-19 e hipoxemia. La aspartato aminotransferasa y la bilirrubina total fueron predictoras de mortalidad en estos pacientes, por lo que su medición al ingreso es una práctica razonable. Es necesario avanzar en la incorporación de estos marcadores en modelos predictivos de mortalidad y en reglas de decisión clínica.
Palabras claves. COVID-19, mortalidad, pruebas de función hepática, transaminasas, bilirrubina, pronóstico.
Abbreviations
ALT: Alanine aminotransferase.
AST: Aspartate aminotransferase.
TB: Total bilirubin.
HUN: Hospital Universitario Nacional.
LDH: Lactate dehydrogenase.
ICU: Intensive care unit.
OR: Odds Ratio.
CI: Confidence interval.
Introduction
COVID-19 is a disease with a strong public health impact that has weakened health care systems and led to an increase in all-cause mortality.1 The natural history of COVID-19 has been continuously elucidated since it was first described in 2019, but to date there is no complete description of this disease. In particular, its clinical features and predictors of poor prognosis are aspects that have been partially studied.2
Liver damage and abnormal liver function tests occur in 20% to 70% of people with COVID-19. However, they are mild to moderate in most cases.3,4 Although the presence of liver injury in COVID-19 patients has not been described as a causal factor of severe outcomes, liver is an organ that may reflect the pathogenic mechanisms of SARS-CoV-2 infection.5,6 Given the above, liver function tests could be considered as potential prognostic markers of morbidity and mortality in COVID-19.7-9
Obtaining accurate prognostic information may help improve the management of patients with COVID-19. However, the validity of liver injury as a prognostic factor in these patients is not entirely clear, as published studies have reported contradictory data.8-10 The aim of the present study was to determine whether alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TB) levels are independent predictors of mortality in adults hospitalized for COVID-19.
Materials and Methods
This was a retrospective cohort study conducted at Hospital Universitario Nacional (HUN), a private quaternary care hospital in Bogota, Colombia. HUN is a teaching hospital that provides health care to adult patients who have health insurance plans. The hospital does not have an emergency department, so most inpatients are referred from other health care facilities. The sampling frame consisted of all patients admitted to the hospital between March 1, 2020 and May 31, 2022. Simple random sampling without replacement was used.11 Inclusion criteria were the presence of COVID-19 and hypoxemia. Exclusion criteria are listed in Table S1.
The following data were collected: age, sex, weight, height, presence of comorbidities, presence of fever, lowest lymphocyte count during hospitalization, blood chemistry tests on admission (lactate dehydrogenase – LDH, D-dimer and alkaline phosphatase levels). In addition, admission ALT, AST and TB levels (defined as levels obtained in liver function tests performed within the first 72 hours of admission), and the highest ALT, AST and TB levels during hospitalization (peak values) were collected. Finally, information about in-hospital mortality (primary outcome) and length of hospital stay, need for intensive care unit (ICU) admission and need for invasive mechanical ventilation (secondary outcomes) were collected.
Statistical analysis and sample size
The mortality (primary outcome) reported in the first 187 patients with COVID-19 enrolled in HUN (15.9%) was used to determine the sample size. Using the normal approximation to the binomial distribution of the outcome and taking into account the frequency of the primary outcome mentioned above, an OR of 1.75 as the minimum detectable effect, a statistical power of 80%, a loss rate of up to 10% and an alpha error for a significance level of 0.05 (two-tailed test), a sample size of 698 patients was calculated.
Data are described using absolute frequencies and percentages for qualitative variables, and medians and interquartile ranges for quantitative variables, as the data showed a non-normal distribution. The normality of the data was assessed using histogram analysis and the Shapiro-Wilk test. Outliers were also identified.
Inferential analysis was performed by hypothesis testing for both the primary outcome (death) and secondary outcomes. Exploratory bivariate analysis was performed using the chi-square test or Fisher’s exact test for categorical variables (all non-ordinal), and the Wilcoxon rank sum test for quantitative variables.
For multivariate analysis, adjustment variables were selected based on the analysis of pathogenic and pathophysiological mechanisms in COVID-19 leading to death and liver injury. These variables were analyzed by a directed acyclic graph (Figure S1) and the those defined as the minimal variables necessary for control of confounding were selected using the online tool DAGitty.12 In addition, the evidence supporting these covariables as independent prognostic factors for mortality was considered as a second criterion for the selection of variables. Thus, age,13 presence of diabetes mellitus,14,15 presence of fever during hospitalization,16-18 lowest lymphocyte count during hospitalization,19,20 D-dimer level on admission21,22 and LDH level on admission were selected as adjustment variables.20,23 Body mass index was not considered because a high proportion of participants (38%) did not have this information recorded.
A logistic regression model was then constructed for dichotomous response variables for the primary outcome (death) and secondary outcomes (ICU admission and need for invasive mechanical ventilation). Multiple linear regression was performed for the outcome of length of stay. The regression was modeled independently for each of the three variables of interest (ALT, AST and TB levels at their highest value during hospitalization) along with the six adjustment variables mentioned above and the Odds Ratio (OR) was calculated. A level of significance of 0.05 was considered in all cases. Data imputation (for n=704) was used in the multivariate analysis, but a sensitivity analysis was also performed using only patients with complete data. The rejection of the null hypothesis in both models (with data imputation and sensitivity analysis) was used as the acceptance criterion for the association between biomarker and outcome; if this criterion was not met, the association was considered inconclusive.
The imputation procedure was performed using multiple data imputation under the assumption of missing at random (MAR). The calculation was performed for 10 imputed data sets using the normal multiple regression method, assuming a normal distribution of the data. Data imputation was performed for the following variables: AST, ALT and total bilirubin levels during hospitalization, lowest lymphocyte count during hospitalization, D-dimer level at admission, and LDH level at admission.
Data were collected and managed using the REDCap data capture software tool, and data analysis was carried out using STATA software version 17.0 (StataCorp LLC, College Station, TX, USA).
The study was approved by the HUN Ethics Committee. It was conducted in accordance with the tenets of the Declaration of Helsinki. Data were collected from medical records and patient anonymity was maintained at all times. It is also presented in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations for reporting cohort studies.24
Results
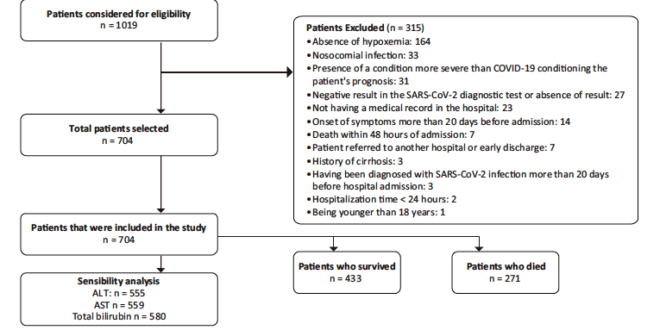
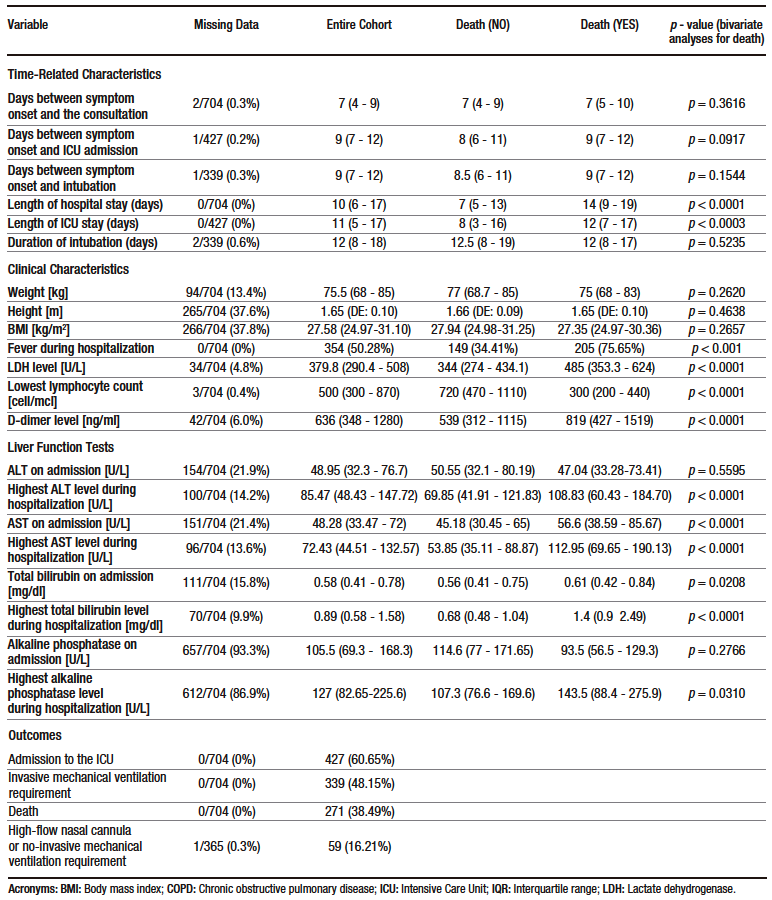
A total of 1019 patients were evaluated for eligibility. Of these, 704 were included in the study. The patient selection process, including reasons for exclusion, is shown in Figure 1. Descriptive data of the sample are presented in Table 1 and Supplementary Table S2. The median age of the participants was 61 years (interquartile range [IQR]: 50 – 69.5 years), and 66.6% were men. Hypertension (41.6%) and diabetes mellitus (20.2%) were the most frequent comorbidities. The median time from symptom onset to consultation was 7 days (IQR: 4 – 9) and the median length of hospital stay was 10 days (IQR: 6 – 17). Furthermore, 60.7% were admitted to the ICU, 48.1% required invasive mechanical ventilation and 38.5% died.
Figure 1. Flowchart of the patient selection process. Information on the reasons for exclusion and the total number of patients included for analysis is also presented
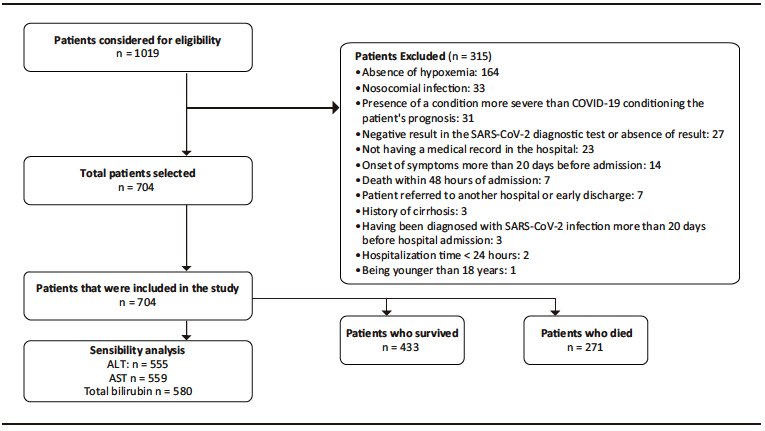
Table 1. Descriptive statistics of the sample and results of the bivariate analysis performed to determine the association between death (primary outcome) and sociodemographic, clinical, and laboratory results variables
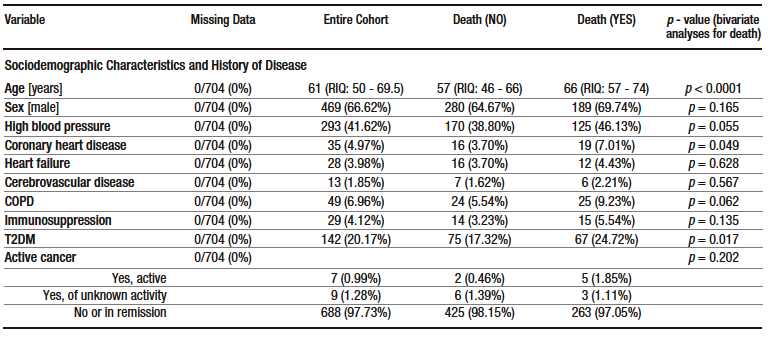
Table 2 shows the proportion of elevated ALT, AST and TB levels according to different cut-off points.
On admission, more than 60% of patients had elevated AST and ALT levels, but only a few of them had severe elevations. On the contrary, only 8.3% of patients had elevated TB levels on admission. Similarly, based on the median ALT, AST and TB levels on admission, the first two were slightly increased, while the latter was within the normal range (see Table 1). In addition, all cases of hyperbilirubinemia were caused by elevated direct bilirubin levels. The frequency of elevated ALT, AST and TB levels was higher in the group of patients who were admitted to the ICU (see Supplementary Table S3).
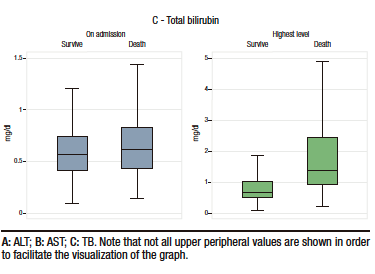
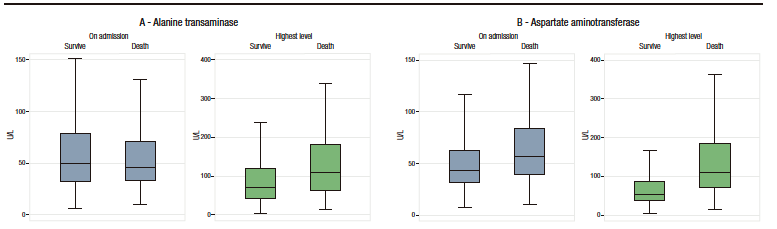
Box plots of these liver injury markers according to the occurrence of the primary outcome (i.e., death) are shown in Figure 2. Descriptive analysis suggests that there were no differences between groups in ALT levels at admission, but that AST and TB levels were higher in patients who died. The levels of these three markers of liver injury during hospitalization were higher in the group of patients who died. (see Table 1 and Figure 2).
Table 2. Proportion of elevated ALT, AST and TB levels according to three cut-off points
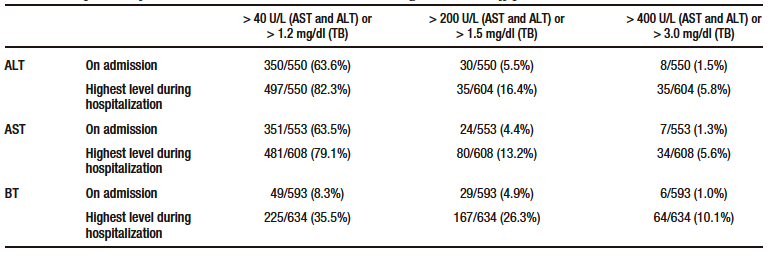
Figure 2. Box plots of both levels on admission and highest value during hospitalization of the markers of liver injury analyzed according to the occurrence of the primary outcome (death)
The following variables were associated with mortality in bivariate analysis: age, presence of coronary heart disease, presence of type 2 diabetes mellitus, presence of fever during hospitalization, elevated LDH and D-dimer levels, lowest lymphocyte count during hospitalization, AST level at admission, TB level at admission, highest ALT level during hospitalization, highest AST level during hospitalization, and highest TB level during hospitalization (see Table 1).
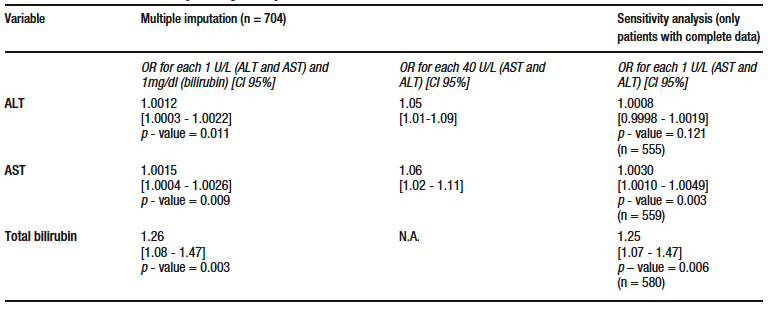
According to the results of multivariate analysis, for each 40 U/L increase in AST level, there was an OR of 1.06 (95% CI: 1.02-1.11, p – value = 0.009), and for each 1mg/dl increase in TB level, an OR of 1.26 (95% CI: 1.08 – 1.47, p – value = 0.003) in the model in which data imputation was performed. This finding was consistent with the results of the sensitivity analysis. ALT level was also significantly associated with mortality in the data imputation model, with an OR of 1.05 (95% CI: 1.01-1.09, p – value = 0.011) for each 40 U/L increase. However, this association was no longer significant in the model where the sensitivity analysis was conducted (p – value = 0.121). Similarly, the association was not significant in the sensitivity analysis after eliminating outlier data according to standardized residuals and residual standard deviation; there were no leverage values. The models for each marker met the goodness-of-fit (Hosmer-Lemeshow > 0.05 in all models) and linearity criteria (STATA link test with a hatsq p – value > 0.05 in all models); no collinearity was found and the data were consistent in the sensitivity analysis after eliminating outlier residuals. The results of the multivariate analysis are shown in Table 3.
Table 3. Multivariate model for the primary outcome (death)
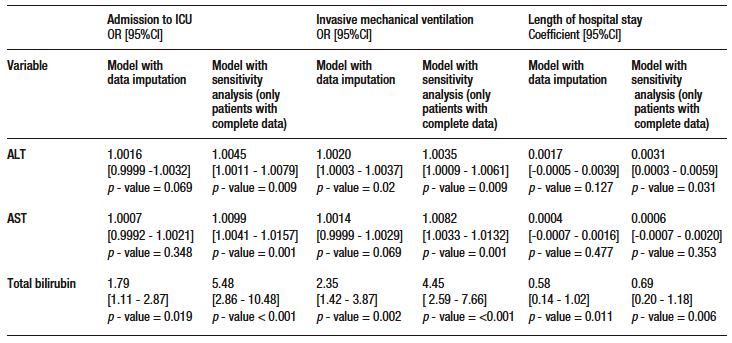
Of the remaining variables in the model, age, presence of fever during hospitalization and lowest lymphocyte count were significantly associated with mortality (see Supplementary Table S4). Regarding secondary outcomes, TB was significantly associated with ICU admission, invasive mechanical ventilation and length of hospital stay. ALT was significantly associated with the need for invasive mechanical ventilation. No significant associations were found between AST and any of the secondary outcome. The models evaluated met the linearity criteria, no collinearity was found, and they had good discriminatory capacity. Data on the association of AST, ALT and TB levels with secondary outcomes are shown in Supplementary Table S5.
Discussion
The present study sought to determine the prognostic value of serum AST, ALT and TB levels in predicting death in patients hospitalized for COVID-19. With a mortality rate of 38%, elevated AST and TB levels were found to be independently associated with mortality. TB level was also associated with ICU admission, need for mechanical ventilation, and length of hospital stay. The results for ALT were inconsistent, so even if the data reported in this study suggest that there is an association between mortality and ALT levels in these patients, it is not possible to draw such a conclusion.
Similarly, it was found that more than 60% of patients had elevated serum ALT and AST levels on admission, although in most of them these elevations were mild. In contrast, only 8% had elevated TB levels on admission. Reports in the literature regarding the frequency of elevated levels of these markers are highly variable. For example, studies have reported that elevated AST and ALT levels are observed in 10% to 60% and 8% to 60% of patients with severe COVID-19.4,10,25-28 The frequency of elevated transaminases and TB found in the present study is higher than that reported in the literature. This may be explained by the fact that our sample tended to have a more severe form of the disease. When we analyze the elevation of ALT, AST and TB levels according to ICU admission or not, those who were not admitted to the ICU had levels closer to those reported by other studies. Ethnic variability in normal values and the concomitant presence of chronic liver disease could also explain the differences.
AST and TB levels were significantly associated with mortality in the present study, while ALT levels are suggested, but not conclusively, as predictors of death. These findings are consistent with what has been reported in some studies, but differ from what has been described in others. This is explained by the fact that the studies conducted on this topic are highly heterogeneous in terms of the characteristics of the populations and the quality of the methodologies used, and therefore the results vary widely. However, they have tended to show a positive association between liver injury and the occurrence of adverse outcomes in COVID-19 patients.29-31
Meta-analyses of studies on this topic have reported results supporting the association of liver injury with death and other adverse outcomes. For example, according to the systematic review by Radivojevic et al., 13 of the included studies found statistical significance in favor of considering transaminases as predictors of death.32 These findings are consistent with those described in other meta-analyses.33-37
Liver damage in COVID-19 can be caused directly or indirectly by the virus.5,6,38 Direct damage is not the most relevant damage mechanism. On the other hand, indirect damage seems to be the most important because it involves a mechanism of immune dysfunction that includes cytokine storm, macro- and micro-thrombotic phenomena, and damage caused by other dysfunctional organs. Liver injury induced by drugs used in the management of COVID-19 such as tocilizumab or remdesivir also plays an important role.5,6,38
Regarding the association between mortality and other variables, we found a significant association with age and low lymphocyte count, a finding that has been extensively reported in the literature. The presence of fever during hospitalization was also associated with death. In this regard, some exploratory studies have described the development of fever during hospitalization as a marker of severity in COVID-19 patients.16-18 Thus, this finding suggests that it may be a promising prognostic factor in this population that requires further research.
Strengths of this study include its sample size and the fact that it was calculated for a statistical power of 80% and a pre-defined minimum OR. In addition, the selection of covariates was based on the COVID-19 mechanisms of injury and prognostic markers reported in the literature, which strengthens the external validity of the study. Similarly, the selection of death as the primary outcome reduced the risk of misclassification bias and co-intervention bias, which would be higher if outcomes such as ICU admission or need for invasive mechanical ventilation would have been chosen as primary outcome.
Regarding its limitations, since the hospital does not have an emergency department and patients are referred from other hospitals, the study may have a referral bias that could affect its external validity, as it is possible that only patients with severe COVID-19 were included in the sample. On the other hand, the retrospective nature of the study implies several limitations, including the lack of knowledge about the patients’ history of liver disease such as metabolic steatotic liver disease, viral hepatitis or alcoholic liver disease, which are potential confounders that could not be controlled. The lack of information on medications and other interventions received during hospitalization hinders the explanatory component of this work. In addition, there was a non-negligible loss of data. Multiple imputation was used to address this problem; this strategy seemed to have a partially acceptable performance, since the results of the sensitivity analyses were consistent with those of the imputed data analyses for AST and TB, but not for ALT.39
Conclusion
Liver injury, as determined by AST, ALT and TB levels, is highly frequent but mild in hospitalized patients with severe COVID-19. AST and TB are independently associated with mortality and should be considered as prognostic markers for mortality in these individuals. In addition, ALT is a possible prognostic marker for mortality. In view of the above, the measurement of AST and TB to evaluate the risk of death in these patients seems a reasonable practice. In addition, the usefulness of these markers in predicting death in COVID-19 is likely to be enhanced if they are included in a prognostic model validated in different populations that considers variables that strengthen its predictive capacity.
Acknowledgments
We thank Aranza Helena Tafur, Emily Caicedo, Juan Diego Kalmar and Manuel Bejarano for their contribution to the data collection.
Consent for Publication. Anonymized data were used for the elaboration of this article, which did not distort its scientific value.
Intellectual Property. The authors declare that the data, figures and tables that appear in this article are original and were made in their belonging institutions.
Funding. The authors declare that there were no external sources of funding.
Conflict of Interest. The authors declare that they have no conflicts of interest related to this article.
Copyright
© 2024 Acta Gastroenterológica latinoamericana. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license, which allows non-commercial use, distribution, and reproduction, provided the original author and source are acknowledged.
Cite this article as: Rodríguez-Gutiérrez A F, Duarte-Calderón C A, Moreno López S M. Elevation of Aminotransferases and Total Bilirubin Levels as Prognostic Markers of Mortality in Adults Hospitalized for COVID-19. A Cohort Study. 2024;54(1):42-55. https://doi.org/10.52787/agl.v54i1.390
References
- Haldane V, De Foo C, Abdalla SM, Jung AS, Tan M, Wu S, et al. Health systems resilience in managing the COVID-19 pandemic: lessons from 28 countries. Nat Med. 2021 May 17; 27(6):964-80. Available from: https://www.nature.com/articles/s41591-021-01381-y
- Burn E, Tebé C, Fernandez-Bertolin S, Aragon M, Recalde M, Roel E, et al. The natural history of symptomatic COVID-19 during the first wave in Catalonia. Nat Commun. 2021 Feb 3;12
(1). Available from: https://pubmed.ncbi.nlm.nih.gov/33536436/ - Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020 Oct;72(4). Available from: https://pubmed.ncbi.nlm.nih.gov/32725890/
- Berenguer J, Ryan P, Rodríguez-Baño J, Jarrín I, Carratalà J, Pachón J, et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020 Nov;26(11). Available from: https://pubmed.ncbi.nlm.nih.gov/32758659/
- Marjot T, Webb GJ, Barritt AS, Moon AM, Stamataki Z, Wong VW, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021 May;18(5). Available from: https://pubmed.ncbi.nlm.nih.gov/33692570/
- Halina Cichoż-Lach AM. Liver injury in the era of COVID-19. World J Gastroenterol. 2021 Feb 7;27(5):377. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7856845/
- Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020 Oct;72(4). Available from: https://pubmed.ncbi.nlm.nih.gov/32725890/
- Ma GG, Shen YX, Wu L, Luo Z, Zhu CW, Chen SY, et al. Effect of liver injury on prognosis and treatment of hospitalized patients with COVID-19 pneumonia. Annals of translational medicine. 2021 Jan;9(1). Available from: https://pubmed.ncbi.nlm.nih.gov/33553303/
- Zhang L, Hou J, Ma FZ, Li J, Xue S, Xu ZG. The common risk factors for progression and mortality in COVID-19 patients: a meta-analysis. Arch Virol;1. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8017903/
- Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020 Oct;72(4). Available from: https://pubmed.ncbi.nlm.nih.gov/32725890/
- Gutiérrez HA. Estrategias de muestreo: diseño de encuestas y estimación de parámetros. Facultad de Estadística, Universidad Santo Tomás; 2009. Available from: https://books.google.com/books/about/Estrategias_de_muestreo.html?id=AXrUswEACAAJ
- Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package “dagitty.” Int J Epidemiol. 2016 Dec 1;45(6). Available from: https://pubmed.ncbi.nlm.nih.gov/28089956/
- Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020 Dec;35(12). Available from: https://pubmed.ncbi.nlm.nih.gov/33289900/
- Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020 Jul;14(4). Available from: https://pubmed.ncbi.nlm.nih.gov/32408118/
- Schlesinger S, Neuenschwander M, Lang A, Pafili K, Kuss O, Herder C, et al. Risk phenotypes of diabetes and association with COVID-19 severity and death: a living systematic review and meta-analysis. Diabetologia. 2021 Jul; 64(7). Available from: https://pubmed.ncbi.nlm.nih.gov/33907860/
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7;323(13). Available from: https://pubmed.ncbi.nlm.nih.gov/32091533/
- Tharakan S, Nomoto K, Miyashita S, Ishikawa K. Body temperature correlates with mortality in COVID-19 patients. Crit Care. 2020 Jun 5;24(1). Available from: https://pubmed.ncbi.nlm.nih.gov/32503659/
- Choron RL, Butts CA, Bargoud C, Krumrei NJ, Teichman AL, Schroeder ME, et al. Fever in the ICU: A Predictor of Mortality in Mechanically Ventilated COVID-19 Patients. J Intensive Care Med. 2021 Apr;36(4). Available from: https://pubmed.ncbi.nlm.nih.gov/33317374/
- Henry B, Cheruiyot I, Vikse J, Mutua V, Kipkorir V, Benoit J, et al. Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: a meta-analysis. Acta Biomed. 2020 Sep 7;91(3). Available from: https://pubmed.ncbi.nlm.nih.gov/32921706/
- Melo AKG, Milby KM, Alma C, Acpn P, Santos RRP, Rocha AP, et al. Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis. PLoS One. 2021 Jun 29;16(6). Available from: https://pubmed.ncbi.nlm.nih.gov/34185801/
- Zhang L, Hou J, Ma FZ, Li J, Xue S, Xu ZG. The common risk factors for progression and mortality in COVID-19 patients: a meta-analysis. Arch Virol;1. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8017903/
- Gungor B, Atici A, Baycan OF, Alici G, Ozturk F, Tugrul S, et al. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: A systematic review and meta-analysis. Am J Emerg Med. 2021 Jan;39. Available from: https://pubmed.ncbi.nlm.nih.gov/33069541/
- Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am J Emerg Med. 2020 Sep;38(9). Available from: https://pubmed.ncbi.nlm.nih.gov/32738466/
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007 Oct 16;147(8). Available from:
https://pubmed.ncbi.nlm.nih.gov/17938389/ - Cho J, Lee J, Sia CH, Koo CS, Tan BY, Hong W, et al. Extrapulmonary manifestations and complications of severe acute respiratory syndrome coronavirus 2 infection: a systematic review. Singapore Med J. 2021 Sep 21; Available from: https://pubmed.ncbi.nlm.nih.gov/34544216/
- Bzeizi K, Abdulla M, Mohammed N, Alqamish J, Jamshidi N, Broering D. Effect of COVID-19 on liver abnormalities: a systematic review and meta-analysis. Sci Rep. 2021 May 19;11(1). Available from: https://pubmed.ncbi.nlm.nih.gov/34012016/
- Chen Z, Peng Y, Wu X, Pang B, Yang F, Zheng W, et al. Comorbidities and complications of COVID-19 associated with disease severity, progression, and mortality in China with centralized isolation and hospitalization: A systematic review and meta-analysis. Frontiers in public health. 2022 Aug 16;10. Available from: https://pubmed.ncbi.nlm.nih.gov/36052001/
- Laali A, Tabibzadeh A, Esghaei M, Yousefi P, Soltani S, Ajdarkosh H, et al. Liver Function Tests Profile in COVID-19 Patients at the Admission Time: A Systematic Review of Literature and Conducted Researches. Adv Biomed Res. 2020 Dec 23;9. Available from: https://pubmed.ncbi.nlm.nih.gov/33912490/
- Cattelan AM, Di Meco E, Trevenzoli M, Frater A, Ferrari A, Villano M, et al. Clinical characteristics and laboratory biomarkers changes in COVID-19 patients requiring or not intensive or sub-intensive care: a comparative study. BMC Infect Dis. 2020 Dec 9;20(1). Available from: https://pubmed.ncbi.nlm.nih.gov/33297986/
- Snipelisky D, Johnson R, Prasad R, Lakhani B, Ellington J. Characteristics and Outcomes Based on Perceived Illness Severity in SARS-CoV-2. South Med J. 2020 Dec;113(12). Available from: https://pubmed.ncbi.nlm.nih.gov/33263129/
- Galiero R, Pafundi PC, Simeon V, Rinaldi L, Perrella A, Vetrano E, et al. Impact of chronic liver disease upon admission on COVID-19 in-hospital mortality: Findings from COVOCA study. PLoS One. 2020 Dec 10;15(12). Available from: https://pubmed.ncbi.nlm.nih.gov/33301529/
- Radivojevic A, Aa AJ, Ravanavena A, Ravindra C, Igweonu-Nwakile EO, Ali S, et al. A Systematic Review of SARS-CoV-2-Associated Hepatic Dysfunction and the Impact on the Clinical Outcome of COVID-19. Cureus. 2022 Jul 14;14(7). Available from: https://pubmed.ncbi.nlm.nih.gov/35974857/
- Cao B, Jing X, Liu Y, Wen R, Wang C. Comparison of laboratory parameters in mild vs. severe cases and died vs. survived patients with COVID-19: systematic review and meta-analysis. J Thorac Dis. 2022 May;14(5). Available from: https://pubmed.ncbi.nlm.nih.gov/35693606/
- Harapan H, Fajar JK, Supriono S, Soegiarto G, Wulandari L, Seratin F, et al. The prevalence, predictors and outcomes of acute liver injury among patients with COVID-19: A systematic review and meta-analysis. Rev Med Virol. 2022 May;32(3). Available from: https://pubmed.ncbi.nlm.nih.gov/34643006/
- Mohammed SA, Eid KM, Anyiam FE, Wadaaallah H, Muhamed MAM, Morsi MH, et al. Liver injury with COVID-19: laboratory and histopathological outcome-systematic review and meta-analysis. Egyptian liver journal. 2022;12(1). Available from: https://pubmed.ncbi.nlm.nih.gov/35096428/
- Sotgia S, Mangoni AA, Mellino S, Cangemi M, Paliogiannis P, Fois AG, et al. A meta-regression study of the clinical significance of serum aminotransferases in COVID-19. Eur Rev Med Pharmacol Sci. 2021 Nov;25(21). Available from: https://pubmed.ncbi.nlm.nih.gov/34787885/
- Zahedi M, Yousefi M, Abounoori M, Malekan M, Tajik F, Heydari K, et al. The Interrelationship between Liver Function Test and the Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Iran J Med Sci. 2021 Jul;46(4). Available from: https://pubmed.ncbi.nlm.nih.gov/34305236/
- Kariyawasam JC, Jayarajah U, Abeysuriya V, Riza R, Seneviratne SL. Involvement of the Liver in COVID-19: A Systematic Review. Am J Trop Med Hyg. 2022 Feb 24;106(4). Available from: https://pubmed.ncbi.nlm.nih.gov/35203056/
- Morris AH, Ioannidis JPA. Limitations of Medical Research and Evidence at the Patient-Clinician Encounter Scale. Chest. 2013 Apr;143(4):1127. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3616682/
Appendix
Figure S1. Directed acyclic graph used for the selection of the adjustment variables of the model. Organ damage (measured by means of lactate dehydrogenase level), diabetes mellitus, age, immunothrombosis (measured using D-dimer level), lymphopenia and obesity were defined as necessary adjustment variables; however, obesity was ruled out due to the high proportion of missing data for this variable in the sample
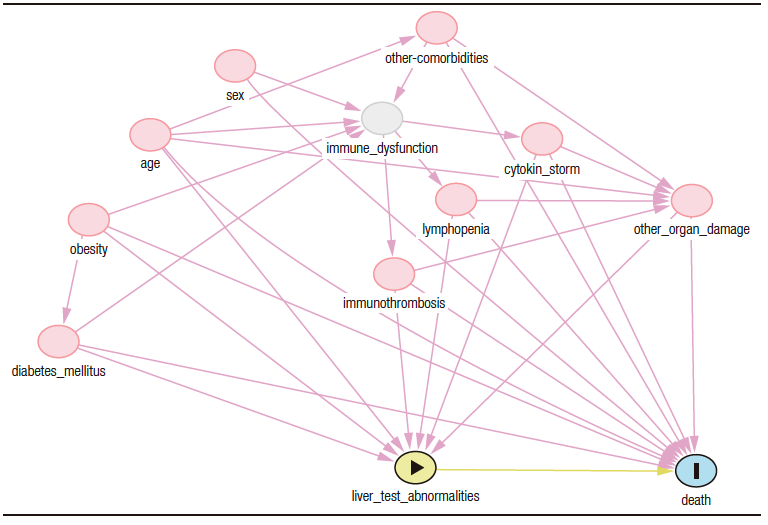
Table S1. Inclusion and exclusion criteria.
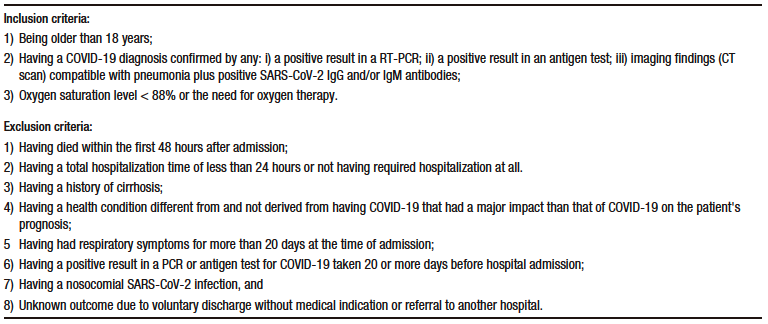
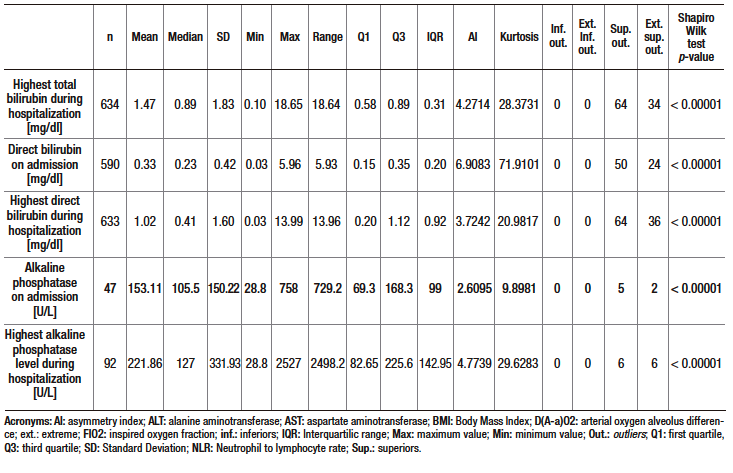
Table S2. Descriptive analysis (central tendency, dispersion, shape and asymmetry) and normality test of quantitative variables
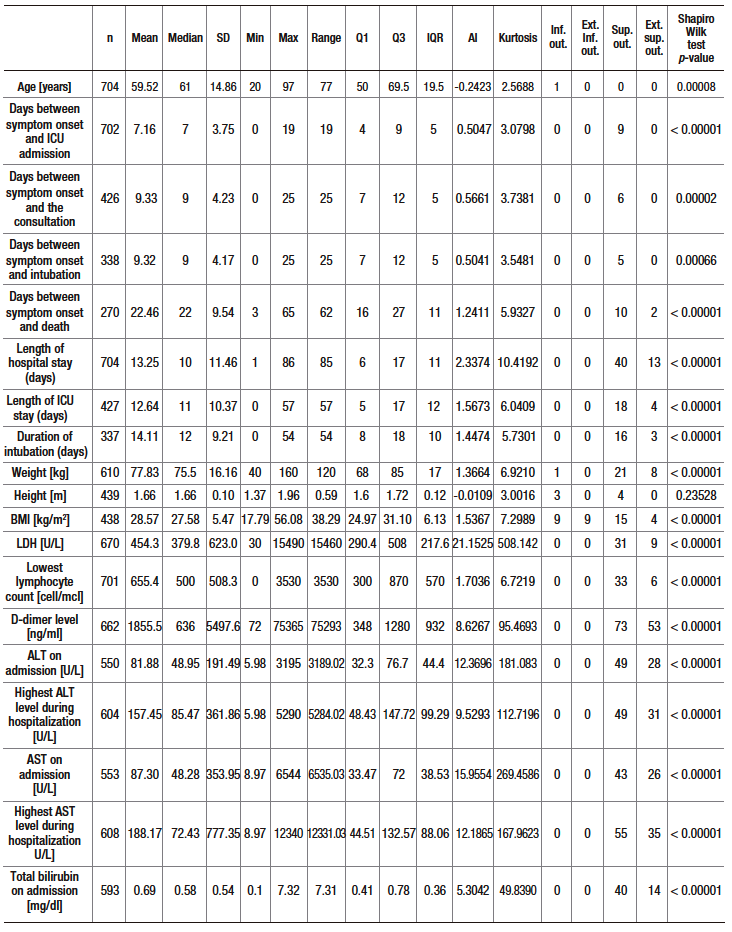
Table S3. Proportion of elevated ALT, AST and TB levels according to three cutoff points depending on ICU admission or not
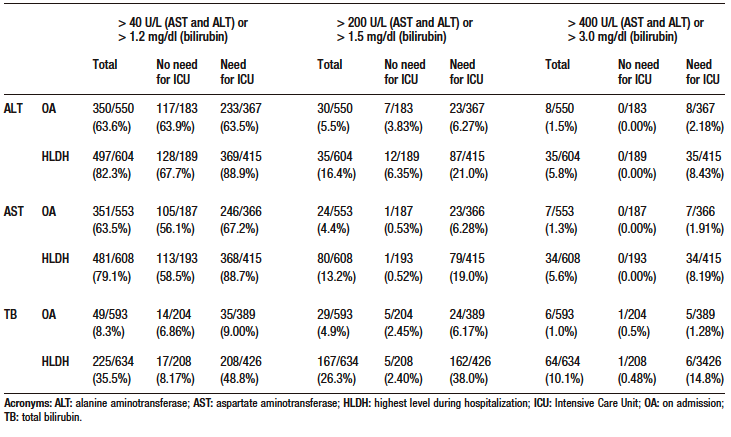
Table S4. Odds ratio and p-value of co-variables in multivariate models for death outcome with multiple imputation and sensitivity analysis
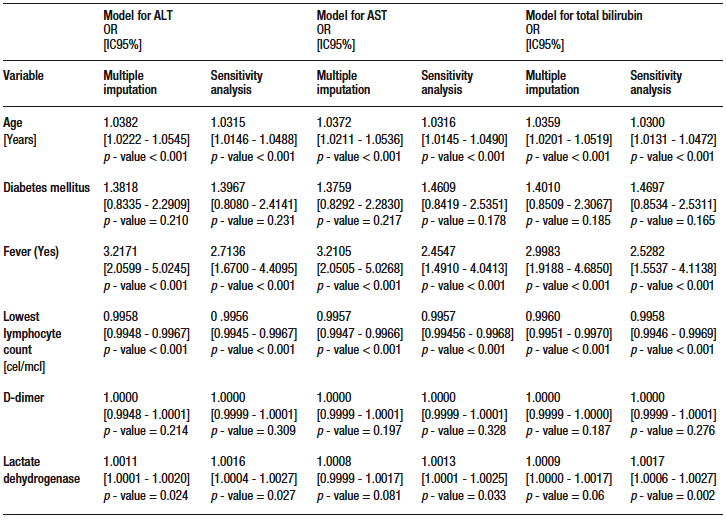
Table S5. Multivariate analysis models (multiple data imputation and sensitivity analysis) for secondary outcomes
Correspondence: Andrés Fernando Rodríguez-Gutiérrez
Email: afrodriguezg@gmail.com
Acta Gastroenterol Latinoam 2024;54(1):42-55
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE