Federico Piñero,1 Sebastián Marciano,2 Margarita Anders,3 Federico Orozco Ganem,3 Alina Zerega,4 Josemaría Menéndez,5 Manuel Mendizábal,1 Matías Tisi Baña,6 Octavio Gil,4 Solange Gerona,5 Eduardo de Santibañes,2 Ricardo Mastai,3 Adrián Gadano,2 Marcelo Silva1
1 Hepatology and Liver Transplant Unit. Hospital Universitario Austral. Pilar. Buenos Aires, Argentina.
2 Liver Transplant Program. Hospital Italiano de Buenos Aires. Ciudad Autónoma de Buenos Aires, Argentina.
3 Liver Transplant Unit. Hospital Alemán, Ciudad Autónoma de Buenos Aires, Argentina.
4 Liver Transplant Unit. Sanatorio Allende. Provincia de Córdoba, Argentina.
5 Liver Transplant Program. Hospital Militar-Hospital de Clínicas. Uruguay.
6 Internal Medicine, Biostatistics. Hospital Universitario Austral. Ciudad Autónoma de Buenos Aires, Argentina.
Acta Gastroenterol Latinoam 2016;46: 300-309
Recibido: 12/01/2016 / Aprobado: 14/03/2016 / Publicado en www.actagastro.org el 01/01/2017
Summary
Recurrent hepatocellular carcinoma (HCC) after liver transplantation (LT) has been related with dismal prognosis. The aim of this study was to describe the treatments performed after HCC recurrence in 2 Latin-American countries, and to identify significant factors related to post progression survival. Methods. From 803 adult transplanted patients in 5 LT centers from Argentina and Uruguay (2005-2011), patients with HCC recurrence were included. “Early recurrence” was considered when it occurred during the first year after transplantation. Patients were analyzed according to the treatment introduced after recurrence diagnosis. The post progression survival was compared. Results. Recurrence presented in 20 out of 135 patients (14.8%), 11 (55%) had early recurrences. After recurrence, 14 patients had good performance status and 18 were Child Pugh A. Ten patients received best supportive care (BSC), 10 patients sorafenib, 1 patient local radiotherapy and 1 patient loco regional surgery. Patients with sorafenib had prolonged post progression survival compared to BSC (5.0 months vs 3.0 months; p = 0.04). On multivariate Cox regression analysis, early recurrence was the only factor related to worst survival, hazard ratio = 3.8 (p = 0.01). A survival benefit with sorafenib in patients with late recurrence when compared to early recurrence was observed (16.0 vs 3.0 months, respectively; p = 0.027). Conclusion. although efficacy of Sorafenib for recurrent HCC should be evaluated in a much-needed randomized controlled trial, a time-selection criterion for this treatment should also be assessed.
Key words. Liver, transplantation, cancer, therapeutics.
Sorafenib para el tratamiento de la recurrencia de hepatocarcinoma post trasplante hepático: experiencia sudamericana
Resumen
La recurrencia post trasplante hepático (TH) del hepatocarcinoma (HCC) es un evento de pobre pronóstico. El objetivo de este estudio fue describir los tratamientos implementados luego de la recurrencia del HCC post TH e identificar los factores pronósticos relacionados a la sobrevida post progresión del HCC. Métodos. De 803 pacientes adultos trasplantados de hígado en 5 centros de TH de Argentina y Uruguay (2005-2011), aquellos pacientes con recurrencia post TH de HCC fueron incluidos. La recurrencia “temprana” fue catalogada a aquella ocurrida dentro de los primeros 12 meses post TH. Se comparó la sobrevida post progresión. Resultados. La recurrencia de HCC post TH ocurrió en 20 de 135 pacientes (14,8%), 11 (55%) con recurrencia temprana. Al diagnóstico, 14 pacientes presentaban muy buen performance status y 18 tenían un score de Child Pugh A. Desde la recurrencia, 10 pacientes recibieron soporte terapéutico paliativo, 10 sorafenib, 1 paciente radioterapia local y otro resección quirúrgica. Los pacientes que recibieron sorafenib presentaron mejor sobrevida post progresión comparado con soporte paliativo (5,0 vs 3,0 meses, respectivamente; p = 0,04). En el análisis multivariado de regresión Cox, la recurrencia temprana fue la única variable independientemente asociada con peor sobrevida, (Hazard ratio = 3,8; p = 0,01). Se observó un beneficio terapéutico del sorafenib en pacientes con recurrencia tardía comparada vs temprana (16,0 vs 3,0 meses, respectivamente; p = 0,027). Conclusión. La eficacia terapéutica del sorafenib post trasplante debe ser evaluada en un estudio aleatorizado y controlado, el tiempo de diagnóstico de la recurrencia podría contemplarse como criterio de selección terapéutico.
Palabras claves. Hígado, trasplante, cáncer, tratamiento.
Abbreviations
AE: adverse events.
AFP: alpha-fetoprotein.
BSC: Best Supportive Care.
CI: confidence interval.
CsA: Cysclosporine A.
CT: computerized tomography.
ECOG: East Cooperative Oncology Group.
HCC: hepatocellular carcinoma.
HR: hazard ratio.
iHCC: incidentally found hepatocellular carcinoma.
IR: interquartile range.
LT: liver transplantation.
MELD: model for end-stage liver disease.
MMF: mophetilmicophenolate.
MRI: magnetic resonance imaging.
mTOR: mammalian target of rapamycin inhibitors.
Mvi: microvascular invasion.
NASH: non-alcoholic steato-hepatitis.
RFS: recurrence free survival.
Tac: tacrolimus.
TTD: total tumor diameter.
UCSF: University of California San Francisco.
The Milan criteria have been accepted in order to select the best hepatocellular carcinoma (HCC) liver transplant candidates.1 However, even with its use, recurrence after liver transplantation (LT) may present at rates reported between 10-15% of the cases.2-5 HCC recurrence after LT is a dramatic event with dismal prognosis and leads to death in most cases.2-4 Median overall survival after recurrence has been described ranging from 14 to 24 months.2-4 Aggressive or combined therapeutic approaches such as systemic chemotherapy or radiotherapy did not show a significant survival benefit after recurrence diagnosis.3, 4
Sorafenib, an oral multikinase inhibitor was the first drug to demonstrate a significant survival advantage in patients with advanced HCC in the non-transplant setting, and is now considered the standard of care in this stage of the disease.6, 7 However, there are no randomized control studies supporting the use of this drug after LT, as these patients have been excluded in previous phase II/III trials.7, 8 Recently, different retrospective published series with a small number of patients have shown that sorafenib prolongs survival in patients with HCC recurrence.9-13
On the other hand, some authors have shown that the key prognostic factor of survival after recurrence diagnosis is time from LT to recurrence.14, 15 Recurrence during the first 12 months after LT (“early recurrence”) has been shown to be an independent predictor of worst survival.14
Consequently, the treatment of HCC in the post-transplant setting is therefore still controversial, particularly regarding the timing of the HCC recurrence. The aim of this cohort study was to describe the treatments performed after HCC recurrence, including sorafenib and to identify significant factors related to post progression survival.
Patients and methods
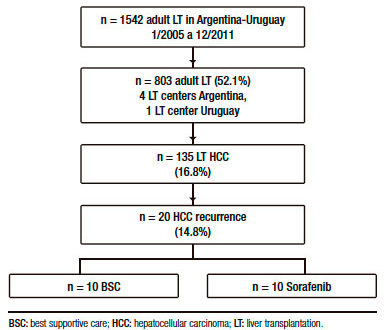
A total of 1542 adult (>17 years of age) LT were performed in Argentina and Uruguay between June 1st 2005 and December 31st 2011. During that period, 803 adult LT were consecutively performed at 4 LT Argentinean centers, the Hospital Universitario Austral, Hospital Italiano from Buenos Aires, Hospital Aleman and the Sanatorio Allende from Cordoba; and at the Hospital Militar- Clínicas from Uruguay. From both cohorts, a total of 135 adult cirrhotic LT patients with confirmed HCC in the explanted liver were analyzed and those patients with recurrent HCC were finally included in the study.
In all centers, prospectively collected data was retrospectively reviewed for recipient characteristics as well as most recent dynamic tumor images and laboratory data prior to transplant.16 The number and corresponding diameters of each HCC nodule seen on Computerized Tomography (CT) or Magnetic Resonance Imaging (MRI) were registered in all cases. In all centers, the HCC standard transplant selection were the Milan criteria, and transplantation for patients exceeding this criteria was discussed in a case-by-case scenario.
All explants from all patients were routinely examined in all centers. The reports of all patients were retrieved from the Pathology files. Microscopic evaluation for each HCC nodule was conducted to characterize tumor characteristics including: number and largest diameter (cm) of HCC nodules, sum of all diameters or total tumor diameter (TTD), presence of microvascular invasion (Mvi), nuclear grade assessed using the modified Edmonson Steiner grading system18 and Up-to 7 criteria.19 These criteria were assessed only in the explanted liver specimen.
Post-LT follow-up visit scheduling was similar in all centers and consisted of one CT or MRI, bone scintigraphy and serum alpha-fetoprotein (AFP) assay every 6 months during the first 3 years in all patients. Tumor recurrence was determined based on imaging criteria16 and serum AFP when there was hepatic involvement or biopsy when isolated extrahepatic metastases of unknown origin were present. Recurrence free survival (RFS) was the period of time calculated in months elapsed from LT to recurrence diagnosis. Recurrence was considered “early HCC recurrence” when it occurred during the first 12 months following transplantation, and “late recurrence” when it occurred later according to previously reported data.14 Performance status according to ECOG and liver function according to the Child Pugh strata was assessed in all patients after recurrence diagnosis.
Patients were analyzed according to the treatment strategy introduced when HCC recurrence was diagnosed. Treatment after recurrence diagnosis either with surgical or loco regional therapies, sorafenib or BSC was reached in a case-by-case basis and was thoroughly discussed in every LT center. Clinical follow-up schedules were performed on an outpatient basis, and included physical examination, laboratory analysis and adverse events (AE) monitoring. BSC was defined as only palliative treatment without any systemic chemotherapy, radiotherapy or surgical resection. In those patients who received sorafenib, date of initiation, starting dose and duration of the drug as well as related AE were registered. Related AE were assessed according to common terminology criteria.20
Induction therapy with interleukin 2 receptor antagonist, basiliximab, and maintenance immunosuppression most frequently used was recorded, such as tacrolimus (Tac), cysclosporine A (CsA) or mammalian target of rapamycin inhibitors (mTOR, sirolimus or everolimus) with or without micophenolate sodium/mophetil (MMF). Although there was no a strict policy of switching from CNI to mTOR immunosuppression, mTORs were considered in all patients, either before or after recurrence diagnosis and was clinically discussed in a case-by-case scenario.
The primary outcome analysis was the post progression survival, which was the time from recurrence diagnosis to death, and was calculated in months. This study was conducted in conformance with the 2008 Helsinki declaration and was approved by each institution board. All study participants, or their legal guardian provided informed written consent prior to study enrollment.
Statistical analysis
Continuous variables were expressed as mean and standard deviation or median and interquartile ranges (IR) according to their distribution, and were compared with Student’s T test or Mann-Whitney U test. Fisher’s exact test (2-tailed) or Chi-Square (X2) test with Yates’ correction was applied to compare categorical data, and were expressed as frequencies and percentages. For the multivariate analysis of death or post progression survival after recurrence diagnosis, Cox regression was applied to the variables that showed risk of death, with a p value of < 0.1 on univariate analysis [Hazard Ratio (HR), Confidence Interval 95% (CI95%)]. Significant values in the multivariate analysis were considered with a p value of < 0.05. Kaplan Meier curves were assessed for the analysis of survival, and the differences between them was analyzed with log-Rank test. In the final model of survival, the premise of proportional hazard assumption is kept in data. Collected data was included in a database and analyzed with R software (version 3.0.1 for Mac).
Results
From 135 adult cirrhotic patients with HCC who were consecutively transplanted with a deceased (n = 132) or living donor (n = 3), 20 (14.8%) presented HCC recurrence (Figure 1).
Patients with recurrence had the following pre transplant baseline characteristics: age 60 years (range: 57-64), MELD score 14 ± 7.11 patients (64.7%) were within Milan criteria and had a median serum AFP 47.5 ng/ml (IR: 11.5-523.7 ng/ml). Explanted liver pathology findings revealed that the median number of HCC nodules was 2.0 (IR: 1.0-4.0), mean total tumor diameter was 6.4 ± 3.6 cm and major nodule diameter 4.8 ± 2.9 cm. Microvascular invasion was present in 55% (n = 11) of these patients, whereas tumors with ≥ III nuclear grade and beyond Up-to 7 criteria were present in 8 (47%) and 11 (55%) patients, respectively (Table 1).
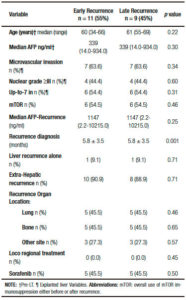
Only 3 out of 20 patients recurred before the year 2008, since sorafenib approval. After recurrence, 14 patients had good performance status (ECOG 0-2) and 18 a well-compensated liver function (Child Pugh A). Median RFS was 10.5 months (IR: 5.0-24.5 months) in a median follow-up period of 16.5 months (IR: 7.5-34.2). Early recurrence presented in 11 patients (55%), while 9 patients had late recurrences. HCC diagnosis after LT was performed with imaging criteria plus AFP in 15 patients (75%) and biopsy in 5 patients (25%). Extra hepatic metastases were present in 18 patients (90%) and only 2 patients had isolated liver involvement (10%) when HCC recurrence occurred. The most frequent recurrence organ location was the liver (n = 12), followed by bones (n = 9), lungs (n = 8) and other sites (n = 6: skin n = 1 and lymph nodes n = 5). Median AFP at time of recurrence diagnosis was 321.5 ng/ml (IR: 21.5-4899.7). Median overall survival and post progression survival were 16.5 months (IR: 8.0-34.2) and 4.0 months (IR: 2.0-7.5), respectively. End-stage and progressive HCC was the main cause of death in all patients.
Basiliximab was administrated in 2 patients, and the most frequent initial maintenance immunosuppression regime was Tac (n = 10, 52%) plus MMF (n = 17, 89%) and steroids (n = 18, 85%). Overall, 12 patients received mTOR immunosuppression (60%), before (n = 7, 58%), or after recurrence diagnosis (n = 5, 42%), either alone or in combination with CNI or MMF. No significant difference in overall median survival in patients receiving or not mTOR immunosuppression was observed (19.0 vs 14 months; p = 0.9).
Treatment performed after recurrence diagnosis
Table 1 shows a descriptive analysis of treatments performed after recurrence. Ten patients received BSC and 10 patients were treated with sorafenib, 1 patient was treated with local radiotherapy and 1 patient with loco regional surgery.
Table 2. Comparative analysis between patients with best supportive care or sorafenib after hepatocellular carcinoma recurrence.
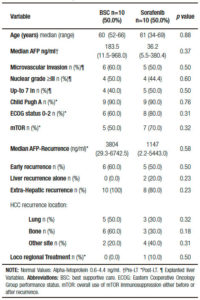
Comparing those patients who received BSC and sorafenib, no significant differences were observed including pre and post-LT patient and tumor characteristics, immunosuppression and early or late recurrences (Table 2). Five out of ten patients treated with BSC had HCC recurrence between 2009 and 2011 (non-historical controls). A similar median RFS between patients with BSC and those treated with sorafenib was observed (9.0 months, (IR: 2.5-20.0) vs 15.0 months (IR: 6.5-27.2 months), respectively; p = 0.65). Although no significant different overall survival was observed (sorafenib 20 months, (IR: 11.0-46.7) vs; BSC 12.5 months (IR: 4.5- 26.0), respectively; p = 0.10), there was a post progression survival benefit in patients treated with sorafenib (5.0 months, (IR: 3.0-16.7) vs 3.0 months, (IR: 0.7-6.0), respectively; p = 0.04) (Figure 2).
Median time from recurrence diagnosis to sorafenib starting dose was 9 weeks (IR: 2-64); 7 patients received 800 mg/day starting dose with an overall 3-months median sorafenib treatment duration (IR: 0.5-6.0). The drug was temporarily interrupted because of AE in 9/10 patients (90%). Most common occurring AE were mild, including diarrhea in 3 patients, fatigue in 4 patients, and hand-foot skin reaction in 1 patient. However, it was completely withdrawn in 2 patients because of severe AE (n = 1 cardiovascular event and n = 1 severe diarrhea).
Figure 2. Kaplan Meier Patient Survival Analysis among those patients with best supportive care vs. sorafenib after recurrence diagnosis (Log-Rank -Mantel Cox- test).
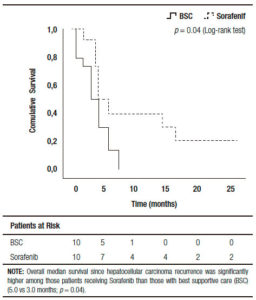
Post progression survival
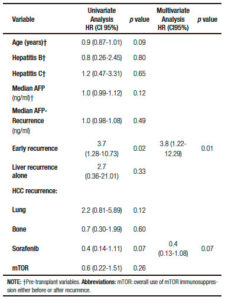
Cox regression univariate analysis showed that related factors with death after recurrence were recipient age, HR = 0.9 (CI95%: 0.87-1.01; p = 0.09), early HCC recurrence, HR = 3.7 (CI95%: 1.28-10.73; p = 0.02) and sorafenib, HR = 0.4 (CI95%: 0.14-1.11; p = 0.07). On multivariate analysis, early HCC recurrence remained the only related factor with death, HR = 3.8 (CI95%: 1.22-12.29; p = 0.01) (Table 3).
Table 3. Cox regression analysis of factors related with death after recurrence diagnosis.
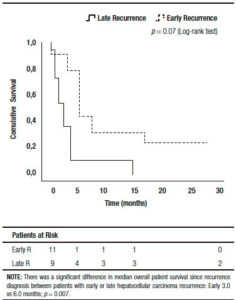
There were no significant base line differences when patients with early or late HCC recurrence were compared (Table 4). A higher survival was observed in patients presenting late recurrences, including median overall survival (37 months, (IR: 24.5-48.5) vs 11 months, (IR: 5.0-14.0) respectively; p = 0.001), and median post progression survival (6.0 months, (IR: 5.0-17.5) vs 3.0 months, (IR: 1.0-4.0) respectively; p = 0.007) (Figure 3).
Figure 3. Kaplan Meier patient survival analysis among those patients with early versus late hepatocellular carcinoma recurrence after liver transplantation (Log-Rank -Mantel Cox- test).
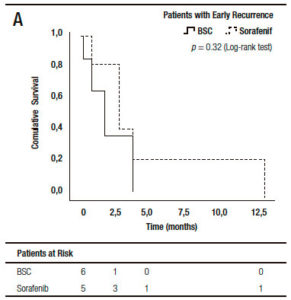
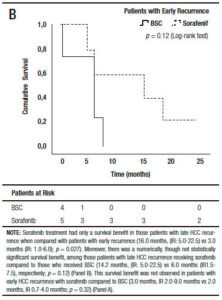
When we analyzed the effect of sorafenib in combination with HCC recurrence presentation, we observed that sorafenib treatment had only a survival benefit in those patients with late recurrence, when compared to early recurrences (16.0 months, (IR: 5.0-22.5) vs 3.0 months, (IR: 1.0-6.0) respectively; p = 0.027 log-rank test). Moreover, there was a numerically, though not statistically significant survival benefit, in those patients with late recurrences receiving sorafenib compared to those with BSC (14.2 months, (IR: 5.0-22.5) vs 6.0 months (IR: 1.5-7.5 months), respectively; p = 0.12). This survival benefit was not observed in patients with early HCC recurrence treated with sorafenib compared to BSC (3.0 months, (IR: 2.0-9.0) vs 2.0 months (IR: 0.7-4.0), respectively; p = 0.32) (Figure 4).
Figure 4. Kaplan Meier patient survival analysis among those patients with early (A) and late hepatocellular carcinoma recurrence (B) with best supportive care or Sorafenib treatment (Log-Rank -Mantel Cox- test).
Table 4. Comparative analysis between patients with early or late recurrent hepatocellular carcinoma.
Discussion
This study reports the factors related to post progression survival after HCC recurrence following transplantation. Our first finding shows that patients with early recurrence had overall worst survival when compared to late recurrences. Second, another factor related to survival was sorafenib treatment. The last finding however, must be validated in large and prospective randomized clinical trials. Meanwhile, there is still no consensus on the management of HCC recurrence following LT. Treatment with sorafenib has been assessed in small retrospective cohort studies9-13, 21, 22 Our results may provide additional information for the field of sorafenib treatment after HCC recurrence. The main finding of our research is that although sorafenib had an overall survival benefit, patients with late recurrence had the best survival benefit with this drug.
Reported mean overall survival in patients treated with sorafenib after recurrence ranges from 5 to 19 months with mean treatment duration with this drug from 2 to 10 months.9-13 There is one recently published retrospective study that has shown that sorafenib (n = 15) prolonged survival compared to BSC (n = 24): 21 vs 12 months, p = 0.0009.9 However, since sorafenib was introduced in 2007, the between subgroups comparison has been carried out with historical controls9 and this probably leaded to a selection bias. Other studies did not compare survival between both treatment options.10-13 In our study, sorafenib had a survival benefit since recurrence diagnosis compared to BSC (5.0 vs 3.0 months; p = 0.04). Median treatment duration (3 months) was comparable with previously reported series.9-13
In the non-transplant setting, overall treatment-related AE with sorafenib are close to 80%, most frequently diarrhea, hand-foot skin reaction, systemic hypertension, alopecia and anorexia.6 In post-LT series, acceptable tolerability and non-serious adverse events were reported, whereas the most frequently occurring AE were diarrhea (13-50%), hand-foot skin reaction (27-60%) and fatigue (20%).9-13 However, in other series, serious AE have been reported.21, 22 In our series, AE presented in 90% of the patients, most of the AE were mild and the drug was withdrawn in 2 patients because of severe AE.
Although we observed that sorafenib had an overall survival benefit, a critical issue to be considered is time from LT to recurrence diagnosis or RFS. Recurrence free survival has been reported to be 8-37 months following transplantation and early recurrence has been found in 28-55% of the patients.9, 13 As we previously highlighted, it has been shown that earlier recurrence is associated with lower survival.14, 15 In our study, patients with early HCC recurrence had lower overall survival and lower survival since recurrence diagnosis (6.0 vs 3.0 months, respectively; p = 0.007). Moreover, early HCC recurrence was the only independent variable associated with survival since recurrence diagnosis in the multivariate analysis, (HR = 3.8; p = 0.002). In one recently published study, although sorafenib had a survival benefit, early HCC recurrence was seen in 42% of the patients with BSC and only in 7% of sorafenib treated patients.9 This kind of imbalance was not observed in our series, regarding RFS between patients with BSC and sorafenib (9.0 vs 15.0 months; p = 0.65), including proportion of patients with early recurrences. As we noted before, sorafenib survival benefit was seen in patients with late HCC recurrence (16.0 vs 3.0 months, respectively; p = 0.027). It seems that a selective criterion for sorafenib treatment could be time from LT to recurrence diagnosis. This earlier HCC recurrence may suggest an aggressive tumor biology or an undiagnosed pre-LT extrahepatic metastasis.14 In this sense, however, no significant differences were observed regarding explanted tumor characteristics, pre and post- LT serum AFP and extrahepatic metastases between those patients with early or late HCC recurrence.
If sorafenib has no survival benefit in those patients with early, but only in late recurrences might not be explained with a lack of anti-neoplastic effect. Probably, those early recurrences suggest occult metastatic foci, and at that moment of transplantation, a more aggressive cancer. Consequently, sorafenib anti-cancer effect might be ineffective at that transplant moment possibly supported by a higher recipients’ immunosuppression state.
Although it was not our primary aim, an additional factor to be considered is immunosuppression. It has been shown that mTOR inhibitors have anti-cancer effects,23 and the combination of mTORs with sorafenib has an hypothetically synergistic approach9, 11, 21, 22 Sixty percent of our patients received mTOR immunosuppression during the post-LT follow-up period, either before or after recurrence. No significant differences, considering mTOR immunosuppression, between patients with BSC or sorafenib were observed. Although in most of the LT centers mTOR immunosuppression was considered either before or after recurrence, a significant survival benefit was not observed in patients who received mTORs. There is still no randomized control trial in which mTOR immunosuppression supports a survival advantage for these patients.
There are several strengths to this study. First, this was a multicenter cohort study from 2 different countries. Second, among all the LT centers, a standardized follow-up and procedures between centers was observed. Third, there were not significant base line patient and tumor characteristics including timing of recurrence diagnosis between patients treated with sorafenib or BSC.
Limitations to this study include: the fact that, although the results are statistically significant, they are based on a retrospective cohort study including a small number of patients. Although the absence of randomization limit the strength of the results, a strict revision of the data was centrally performed, and complete follow-up for the outcomes was assessed. Finally, there is a need for additional tumor biomarkers in the explanted liver analysis in order to select patients for individual treatment options. Prospective randomized and larger series are needed in order to confirm our data, and should include sorafenib therapeutic effect time-dependency alongside a better characterization of the tumor biology using biomarkers.
In summary, our data and other recently retrospective published series suggests that sorafenib treatment might have a survival benefit over BSC for recurrent HCC. However, we observed that patients with early HCC recurrence might not benefit with this treatment. Therefore, time from LT to recurrence diagnosis may be a selection criterion of sorafenib treatment candidates. Nevertheless, safety issues related to sorafenib treatment should be considered in this population. Our results might support the use of this time-selective criterion to design a much-needed randomized controlled trial.
Acknowledgments. We thank Kathleen Dowd and Martín O’Flaherty for their assistance with editing this paper.
Financial support. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest. The authors declare having no conflict of interest to declare related to this research.
Data sharing. No additional data are available.
Referencias
- Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: An evidence-based analysis of 15 years of experience. Liver Transpl 2011; 17: S44-S57.
- Piñero F, Mendizabal M, Casciato P, Galdame O, Quiros R, Bandi J, Mullen E, Andriani O, de Santibañes E, Podestá LG, Gadano A, Silva M. Is recurrence rate of incidental hepatocellular carcinoma after liver transplantation similar to previously known HCC? Towards a predictive recurrence score. Ann Hepatol 2014; 13: 211-218.
- Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger N, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: Patterns and prognosis. Liver Transpl 2004; 10: 534-540.
- Cescon M, Ravaioli M, Grazi GL, Ercolani G, Cucchetti A, Bertuzzo V, Vetrone G, del Gaudio M, Vivarelli M. Prognostic Factors for Tumor Recurrence after a 12-Year, Single-Center Experience of Liver Transplantations in Patients with Hepatocellular Carcinoma. Journal of Transplantation 2010; 2010: 1-8.
- Kneteman N, Livraghi T, Madoff D, de Santibañez E, Kew M. Tools for monitoring patients with hepatocellular carcinoma on the waiting list and after liver transplantation. Liver Transpl 2011; 17: S117–S127.
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle P, Seitz JF, Borbath I, Häussinger D, Moscovici DV, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390.
- Ann-Lii Cheng, Yoon-Koo Kang, Zhendong Chen, Chao-Jung Tsao, Shukui Qin, Jun Suk Kim, Rongcheng Luo, Jifeng Feng, Shenglong Ye, Tsai-Sheng Yang, Jianming Xu, Yan Sun, Houjie Liang, Jiwei Liu, Jiejun Wang, Won Young Tak, Hongming Pan, Karin Burock, Jessie Zou, Dimitris Voliotis, and Zhongzhen Guan. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncology 2009; 10: 25-34.
- Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008; 48: 1312–1327.
- Sposito C, Mariani L, Germini A, Reyes MF, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: A case-control study. Journal of Hepatology 2013; 59: 59-66.
- Vitale A, Boccagni P, Kertusha X, Zanus G, D’Amico F, Lodo E, Pastorelli D, Ramirez R, Lombardi G, Senzolo M, Burra P, Cillo U. Sorafenib for the Treatment of Recurrent Hepatocellular Carcinoma After Liver Transplantation? TPS 2012; 44: 1989-1991.
- Gómez-Martín C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, Herrero I, Matilla A, Sangro B. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl 2011; 18: 45-52.
- Weinmann A, Niederle IM, Koch S, Hoppe-Lotichius M, Heise M, Düber C, Schuchmann M, otto G, Galle P, Wörns M. Sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Digestive and Liver Disease 2012; 44: 432–437.
- Wei-feng Tan, Zhi-quan Qiu, Yong Yu, Rong-zheng Ran, Bing Yi, Wan-yee Lau, Chen Liu, Ying-he Qiu, Fei-ling Feng, Jing-han Wang, Pei-ning Yan, Bai-he Zhang, Meng-chao Wu, Xiang-ji Luo, and Xiao-qing Jiang. Sorafenib extends the survival time of patients with multiple recurrences of hepatocellular carcinoma after liver transplantation. Nature Publishing Group 2010; 31: 1643-1648.
- Toso C, Cader S, Mentha-Dugerdil A, Meeberg G, Majno P, Morard I, Giostra E, Berney T, Kneteman N. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J Hepatobiliary Pancreat Sci 2012; 20: 342-347.
- Woo Young Shin, Kyung-Suk Suh, Hae Won Lee, Joohyun Kim, Taehoon Kim, Nam-Joon Yi, and Kuhn Uk Lee. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl 2010;16: 678-684.
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208-1236.
- Yao FY. Liver Transplantation for Hepatocellular Carcinoma: Beyond the Milan Criteria. American Journal of Transplantation 2008; 8: 1982-1989.
- Villanueva A, Hoshida Y, Battiston C, Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashi M, Kumada H, Thung SN, Bruix J, Newell P, April C, Fan JB, Roayaie S, Mazzaferro V, Schwartz ME, Llovet JM.Combining Clinical, Pathology, and Gene Expression Data to Predict Recurrence of Hepatocellular Carcinoma. Gastroenterology 2011; 140: 1501-1512.
- Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwatrz M, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncology 2009; 10: 35-43.
- Trotti A, Colevas A, Setser A, Rusch V, Jaques D, Budach V, Langer C. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in Radiation Oncology 2003; 13: 176-181.
- Staufer K, Fischer L, Seegers B, Vettorazzi E, Nashan B, and Sterneck M. High toxicity of sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Transplant Int 2012; 25: 1158-1164.
- Zavaglia C, Airoldi A, Mancuso A, Vangeli M, Viganò R, Cordone G, Gentiluomo M, Belli LS. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation. Eur J Gastroenterol Hepatol 2013; 25: 180-186.
- Bhat M, Sonenberg N, Gores GJ. The mTOR pathway in hepatic malignancies. Hepatology 2013; 58: 810-818.
Correspondencia: Federico Piñero
Hospital Universitario Austral
Av Presidente Perón 1500, (B1629HJ) Pilar. Buenos Aires, Argentina
Tel.:(+54) 0230 448-2000
Correo electrónico: fpinerof@cas.austral.edu.ar
Acta Gastroenterol Latinoam 2016;46(4): 300-309
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE