Marcos Cardoso Rios,1 LayanaTyara Sandes Fraga,2 Tereza Virginia Silva Bezerra do Nascimento,2 Ângelo Roberto Antoniolli,3 Divaldo Pereira de Lyra Júnior,1 Alex França2
1 Department of Pharmacy, Laboratory for Teaching and Research in Social Pharmacy.
2 Department of Medicine.
3 Department of Physiology, Federal University of Sergipe UFS, São Cristóvão, SE, Brazil.
Acta Gastroenterol Latinoam 2017;47:14-22
Recibido: 28/09/2015 / Aprobado: 29/09/2016 / Publicado en www.actagastro.org el 05/04/2017
Summary
Recently, pharmacotherapy has been increasingly effective in chronic Hepatitis C due to improved medication and optimized treatment duration. Nevertheless, Hepatitis C treatment in the state of Sergipe mainly consists of dual therapy with interferon (IFN) and ribavirin (RBV) and few studies have evaluated Hepatitis C treatment in Brazil. Thus, the epidemiological profile and treatment response of patients with chronic Hepatitis C treated with IFN and RBV at the University Hospital of Sergipe were evaluated. The medical records of all patients with Hepatitis C who underwent antiviral therapy with IFN and RBV between 2002 and 2012 were reviewed retrospectively. A total of 244 records were analyzed, representing 298 courses of Hepatitis C treatment. On average, 27 patients were treated annually, with a ratio of 1.8:1, being the patients predominantly infected with genotype 1virus (82.0%). Only 6.9% of patients were treated with conventional IFN (3MIU), while 60.3% were treated with alfa peginterferon-2a and 31.0% were treated with alfa peginterferon-2b. Sustained virologic response (SVR) was observed in 38.1% of patients and 36.8% of patients who were PCR-negative at the end of treatment relapsed. SVR rates ranged from 40.8% for naïve patients to 58.3% for previously treated patients with recurrent disease. SVR was higher among non-cirrhotic patients (77.8%) compared with cirrhotic patients (22.2%). The highest SVR rate (83%) was found for naïve, non-cirrhotic patients. Although SVR rates were lower than those found for newer medications, dual therapy with IFN and RBV may be reasonably used to treat chronic Hepatitis C when newer drugs are not available and in special cases.
Key words. Hepatitis C, pharmacotherapy, interferon, ribavirin, treatment.
Interferón y ribavirina para la Hepatitis c crónica: ¿deben ser administrados en la nueva era de tratamiento?
Resumen
Recientemente, la farmacoterapia es cada vez más eficaz en la Hepatitis C crónica debido a la mejor medicación disponible y a la optimización de la duración del tratamiento. Sin embargo, en el estado de Sergipe la terapia utilizada es dual con interferón (IFN) y ribavirina (RBV) y pocos estudios lo han evaluado en Brasil. Así, se evaluó el perfil epidemiológico y la respuesta al tratamiento de los pacientes con Hepatitis C crónica tratados con IFN y RBV en el Hospital Universitario de Sergipe. Se revisaron retrospectivamente las historias clínicas de todos los pacientes con Hepatitis C que se sometieron a la terapia antiviral con IFN y RBV entre 2002 y 2012. En total, se analizaron 244 registros que representan 298 cursos de tratamiento de la Hepatitis C. En media, 27 pacientes fueron tratados al año, con una razón de 1,8 hombres para una mujer, predominantemente infectados con el genotipo 1virus (82,0%). Sólo el 6,9% de los pacientes fueron tratados con IFN convencional (3MIU), mientras que el 60,3% fueron tratados con alfa peginterferon-2a y el 31,0% fueron tratados con alfa peginterferon-2b. Se observó una respuesta virológica sostenida (RVS) en el 38,1% de los pacientes y el 36,8% de los pacientes que fueron PCR-negativos al final del tratamiento presentaron recaída. Las tasas de RVS oscilaron entre 40,8% para los pacientes vírgenes a 58,3% para los pacientes tratados previamente con enfermedad recurrente. La RVS fue mayor entre los pacientes no cirróticos (77,8%) en comparación con los pacientes cirróticos (22,2%). La tasa RVS más alta (83%) se encontró en los pacientes no cirróticos no tratados previamente. Aunque las tasas de RVS fueron inferiores a las encontradas para nuevos medicamentos, la terapia dual con IFN y RBV puede usarse razonablemente para tratar la Hepatitis C crónica en casos especiales o cuando los medicamentos más nuevos no están disponibles.
Palabras claves. Hepatitis C, farmacoterapia, interferón, ribavirina, tratamiento.
Abbreviations
IFN: interferon.
RBV: ribavirin.
PEG-IFN: peginterferon.
SVR: sustained virologic response.
RNA: ribonucleic acid.
IP: protease inhibitor.
RVR: rapid virologic response.
ETR: virologic response at end of treatment.
Rel: relapse.
Hepatitis C is regarded as a public health problem, with an estimated 185 million people diagnosed with the virus, and a further 3 million who are unaware they are infected.1, 2 Approximately 80-85% of cases become chronic, with important clinical consequences such as increased risk of cirrhosis and hepatocellular carcinoma. Thus, adequate and uninterrupted treatment should be carefully administered.3
The aim of drug therapy is eliminate the virus and prevent disease progression. Currently, the best indicator of effective treatment is sustained virologic response (SVR), defined as the absence of detectable viral RNA in serum 12–24 weeks after the end of treatment.4, 5
In recent years, drug therapy in patients with chronic Hepatitis C has achieved increasingly effective results due to improvements in medication and optimized treatment duration.
Drug therapy for Hepatitis C initially consisted of interferon (IFN) monotherapy; however, the rates of SVR achieved were lower than those with subsequent combination therapy with IFN and ribavirin (RBV): 6% versus 31% for a 24-week treatment period and 13% versus 38% for a 48-week treatment period, respectively.6 RBV monotherapy does not result in a satisfactory end of treatment response or SVR.4, 7-8
The addition of polyethylene glycol to the IFN moiety (known as peg interferon) increased the half-life of this drug leading, to prolonged activity and allowing a longer interval between doses. This resulted in improved response to therapy.4, 9-10 Compared with conventional IFN, peg-interferon is associated with a higher response rate in patients, with up to 69% achieving SVR.11 There are two types of peg interferon, alfa2a and alfa2b, which have similar therapeutic efficacy.41, 12 Furthermore, a number of novel protease inhibitors (IPs) have recently been introduced including the first generation IPs telaprevir and boceprevir, and second generation IPs simeprevir, sofosbuvir, nucleotide analogue HCV NS5B polymerase inhibitor, and daclatasvir HCV NS5A replication complex inhibitor.1, 13
In Brazil, where the prevalence of Hepatitis C is 1.38%,14 IFNs (standard and pegylated), RBV, and first-generation IPs are available in the public health system. However, despite access to new drugs, the majority of patients with Hepatitis C receive dual therapy with IFN and RBV. In this study we evaluate the epidemiology, therapy profile, and treatment response of patients with chronic Hepatitis C treated with IFN and RBV in a hospital in Northeast Brazil.
Materials and methods
A cross-sectional review was conducted of the medical records of all Hepatitis C patients who received antiviral therapy with IFN and RBV between the years 2002 and 2012 from the Hepatology Service at the University Hospital of Sergipe in Northeast Brazil. The work was approved by the Research Ethics Committee of the Federal University of Sergipe. The study population included outpatients with chronic Hepatitis C, regardless of sex or race, who began treatment with IFN (conventional or pegylated) and RBV during the study period.
The following epidemiological variables were included: sex, age, skin color, place of residence, profession, comorbidities, disease progression, and possible contagion. To characterize drug therapy, the following data were collected: genotype, histologic evaluation (based on METAVIR classification), medical condition and duration of treatment, antiviral drugs received, changes during drug therapy, adverse events, and medications used to treat adverse events. Antiviral therapy was assigned in accordance with Clinical Protocols and Therapeutic Guidelines for viral Hepatitis C and co-infections from the Brazilian Ministry of Health. The medications administered during treatment were classified according to the Brazilian Common Denomination.
Patients with Hepatitis B and HIV were excluded from the treatment response analysis. Patient response to antiviral treatment was categorized based on the following outcomes defined by the Ministry of Health:4, 5 rapid virologic response (RVR; PCR-negative at week 4); virologic response at end of treatment (ETR; PCR-negative at end of treatment); sustained serologic response (SVR; PCR-negative 6 months post treatment); Relapse (Rel; viral breakthrough).
Data were analyzed using descriptive statistics with SPSS version 20. SVR and medication type were analyzed using the Chi-square test and Pearson’s correlation coefficient. It was considered 95% confidence interval and a value of p < 0.05 was considered significant. Following analysis, the results were expressed as text, graphics, and tables using Microsoft Excel 2010.
Results
Overall 279 patients were identified, 14 of whom were excluded due to co-existing Hepatitis B infection, three cases where it was not used RBV due to chronic renal failure and 21 were excluded as their records could not be found. Thus, 241 patients undergoing a total of 294 courses of treatment from 2002-2012 were analyzed, with an average of 1.2 courses of treatment per patient. An average of 27 patients were treated annually, with the highest numbers treated in 2012 (n = 37) and the lowest in 2009 (n = 19). The ratio of males:females was 1.8:1. Table 1 shows socio-demographic characteristics. The majority of patients (72.8%) lived in Aracaju, the capital of Sergipe, and the most common occupations were trader and retiree (13.8% each).
The main causes of infection or Hepatitis C were (n = 158): history of blood transfusion (44.3% [70]); year of receipt unspecified in most medical records), sexual activity without a condom (43.0 % [68]), intravenous drug use (30.4% [48]), tattoos (15.2% [24]), more than three sexual partners per year (12.7% [20]), health professionals (8.9% [14]), acupuncture (3.2% [5]), and piercings (1.3% [2]). These data were not available in 86 cases (35.3%).
Among the patients treated, 91% (193/212) had comorbidities. Alcohol consumption was the most common (46.7% [99]), followed by arterial hypertension (28.3% [60]), smoking (18.9% [40]), type 2 diabetes mellitus (15.1% [32]), Hepatitis B infection (8% [17]), mental health issues (6.1% [13]), HIV infection (6.1% [13]), thyroid disease (3.3% [7]) and coagulopathy (0.5% [1]).
Table 1. Socio-demographic characteristics of patients treated for Hepatitis C at University Hospital of Sergipe in Northeast Brazil from 2002-2012.

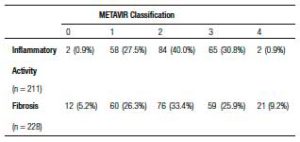
The most common viral genotype in this study was 1B, with a prevalence of 46.6% (102), followed by 1A (32%; 70), and 3A (16.5%; 36). A total of 238 biopsies were analyzed, with inflammatory activity detected in 211 cases and fibrosis in 228.
According to METAVIR classification, the prevalence of advanced fibrosis was 35.1% (80/228 patients), F3 distributed fibrosis was 25.9%, and F4 fibrosis was 9.2%. The results of patients’ pathological examinations are shown in Table 2. In 6 patients out of a total of 27 patients with cirrhosis, cirrhosis was established by clinical, laboratory, endoscopic, or diagnostic imaging. On average, each cirrhotic patient received 2.03 courses of treatment, with 55 courses of treatment received overall. The majority of patients treated (80% [221/276]) were non-cirrhotic. Notably, in 7.4% (22/298) of the charts these data were missing. In addition, 3.6% (8/222) of patients were diagnosed with hepatocellular carcinoma and 4.0% (9/222) died during the study period.
Table 2. Histopathological examination of biopsied tissue specimens from patients treated for Hepatitis C at University Hospital of Sergipe in Northeast Brazil based on METAVIR classification.
In patients receiving IFN, 6.9% (19/271) received conventional IFN (3MUI) and 93.1% (252/271) received peg IFN, of these 60.3% (152/252) received alfa-2a, 31.0% (78/252) received alfa-2b, and data were missing for 8.7% (22/252). Since RBV was used in all cases in which the information was consistent (278/278).
The previous viral load to treatment was analyzed in 210 records, averaging 1.200.000 IU/mL and 5.9 log. The duration of antiviral treatment was 48 weeks in 60.4% (174/288) of cases, 24 weeks in 10.4% (30/288) of cases, and 72 weeks in 4.5% (13/288) of cases. The duration of other treatment courses varied: in 11.3% of the cases the treatment duration was 2-23 weeks, in 10.4% 26-46 weeks, and in 1.0% 51-52 weeks. These data were missing for 2.43% cases (7/288).
Figure 1. Frequency of side effects associated with dmg therapy for Hepatitis C in patients treated at University Hospital of Sergipe in Northeast Brazil from 2002-2012.
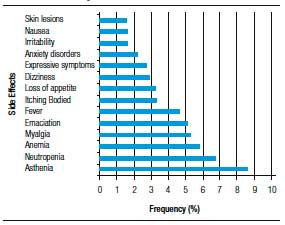
In a number of cases, doses were modified and/or treatment was interrupted. RBV dosage was modified in 18.4% (45/245) of treatment courses and IFN dosage was modified in 6.7% (17/253). RBV treatment was interrupted temporarily in 13.7% (35/256) of cases and IFN was interrupted temporarily in 18.8% (33/165). Of the IFN dose interruptions, 45.5% (15/33) were for alfa-2a, 30.3% (10/33) to alfa-2b, 12.1% (4/33) for unspecified peginterferon, 6.1% (2/33) for standard IFN, and 6.1% (2/33) to unspecified IFN. Treatment was stopped completely in 24.7% (72/291) of cases.
In a number of cases the reasons for treatment discontinuation were reported, these included lack of response to medication, adverse events, and non-compliance. Overall, 11.4% (25/219) of the treatment courses were discontinued due to adverse events and only 1.6% (4/243) of cases reported no adverse events in response to medication. The average number of adverse events was 6.7/treatment course and the range was 0-29. The most common adverse event was asthenia (8.6%). Adverse events were reported in 167 cases with a total of 1501 events, these are summarized in Figure 1. The number of adverse events caused by pegylated versus conventional IFNs were compared, with the average frequency/treatment course of 7.7 for peginterferon alfa-2b and RBV, 6.5 for conventional IFN and RBV, and 5.8 for peginterferon alfa-2a and RBV.
Neutropenia was observed during treatment in 55.1% (134/243) of cases, of these 63.4% (85/134) were associated with peginterferon alfa-2a, 23.1% (31/134) with peginterferon alfa-2b, and 1.5% (2/134) with conventional IFN; in 8.2% (11/134) of cases the type of peg IFN administered was not specified, and in 3.7% (5/134) drug data were not available. Filgrastim was administered in 25.4% (34/134) of cases. In 10.5% (14) of cases the dose of IFN was interrupted, and in 17.2% (23) of cases all treatment was interrupted.
Anemia was observed in 40.7% (99/243) of the cases. In 31.3% of these cases (31/99) erythropoiet in administration was required, in 34.3% (34/99) there was no change in RBV dosage, and in 22.2% (22/99) treatment was discontinued.
Some medications were administered to reduce the effects of adverse reactions in patients receiving antiviral therapy. Overall, 93 different drugs were administered, with an average of 1.47 received per patient. The most frequently administered were: paracetamol (19.8% [45]), loratadine (7.0% [16]), paroxetine (5.7% [13]), vitamin complexes (5.7% [13]), omeprazole (4.8% [11]), diazepam (3.9% [9]), folic acid (3.1% [7]), and scopolamine (2.6% [6]). In 49.3% of treatment courses (112/227) medication was not required for adverse events; and in 23.8% (71/298) of cases these data were not available.
In the majority of cases the patients were treatment-naïve (72.7% [205/282]), for the remainder, 22.7% (64/282) were receiving treatment for the second time, 4.25% (12/282) for the third time, and 0.7% (2/282) for the fourth time; data were not available in 12 cases.
In 109 cases the clinical outcome was inconclusive. In the 189 cases in which clinical outcome could be evaluated, 38.1% (72/189) achieved SVR. Of the 114 patients who were ETR, 63.2% (72) achieved SVR and 36.8% (42) relapsed. The main for patients not achieving ETR were: suspension of treatment due to adverse events (33.3% [25]), lack of response to medication (60% [45]; partial response, null, or breakthrough), and avoidance/ other [6.7% (5)]. Among these non-responders, 61% (54/89) had advanced fibrosis, with 63% of these having F4 fibrosis and 37% F3 fibrosis.
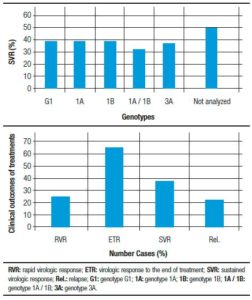
Regarding the type of peginterferon, the SVR rate was 47% (47/100) in patients who used alfa-2a and 29.8% (17/57) associated with alfa-2b (p = 0.031). The SVR rate was 40.8% (56/137) in treatment-naïve patients and 58.3% (7/12) in patients who had relapsed following prior treatment. Among the patients achieving SVR, 87.5% (63/72) were treatment-naïve or had relapsed previously. In 81% of cases (52/64) it was SVR can relate to the type of liver fibrosis. The proportion of patients achieving SVR was higher for those on-cirrhotic (88.5% [46/52]) compared with cirrhotic patients (11.5% [6/52]), with overall rates of 71.8% and 9.4%, respectively. We found that the best outcomes were achieved for treatment-naïve patients who were non-cirrhotic; in these patients the SVR rate was 83% (39/47). Table 3 and Figure 2 summarize results according to the degree of fibrosis, Hepatitis C virus genotype, and medication received.
Table 3. Clinical outcomes of treatment for Hepatitis C at University Hospital of Sergipe in Northeast Brazil from 2002 and 2012.
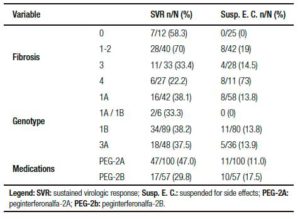
Figure 2. Sustained virologic response according to genotype, medication and clinical outcomes of treatment in patients with Hepatitis C at University Hospital of Sergipe in Northeast Brazil, from 2002-2012.
Discussion
We found that the prevalence of Hepatitis C in males in this study was similar to values cited in the literature.4, 15- 17 This may be associated with a higher exposure of males to risk factors. The age group with the highest prevalence of Hepatitis C in this study was also similar to values cited in the international literature.18, 19 Applying the standard prevalence for anti-HCV positivity during the exposure period, as per Martins et al, the research results, it is suggested that infection has occurred in the distant past.16
Advanced age has been highlighted as one of the most important reasons for failing to achieve SVR. This is because a low glomerular filtration rate can result in low renal clearance of RBV and subsequently high systemic exposure, leading to drastic reductions in dosage, or even discontinuation, of RBV. The identification of patients at a higher risk of developing RBV-associated anemia can also help with patients election and management.20 The highest prevalence of Hepatitis C here was in mixed-race patients, in contrast to Smith et al, this may be due to regional differences.18 There was a low proportion of black patients in this study and they had a lower probability of achieving SVR.21
Among the comorbidities identified in this study, alcoholism can contribute to the pathogenesis of this disease, since alcohol consumption affects certain components of the immune response and can alter the inflammatory response of cytokines, leading to increased viremia.22, 23 Hypertension, diabetes mellitus, and HIV were also found to be associated with Hepatitis C. According to McGowan et al, these comorbidities can have a negative impact on treatment outcomes.24
Nogueira et al. showed that, about 20% of patients treated with IFN and RBV are also treated for hypertension, with 45% of these treatments resulting in severe drug interactions that required intervention.25 This shows that drug-drug interactions should be monitored carefully by healthcare practitioners to ensure patient safety. Patient exposure to pharmacotherapy should be limited if there is a low likelihood of achieving SVR versus a high likelihood of adverse events.
The management of adverse events is essential for patient compliance with treatment. Adverse events have been shown to account for up to 42.8% of cases involving discontinuation of treatment.26-28
The literature shows that changes in hematological parameters are the most commonly reported adverse events associated with Hepatitis C treatment.29 In this study, the rate of anemia was twice as high as that in a study by McHutchison et al, at 40.7% versus 22%.11 In addition, the level of neutropenia was slightly higher than that seen by Nogueira et al, at 55.1% versus 48.0%.25 The appearance of these conditions led to dose modifications for RBV (18.4%) and IFN (6.7%), or dose delays (33.7%), consistent with findings in the literature.28-32
In the present study, the rate of anemia was twice as high as that found by McHutchison et al, at 40.7% versus 22%.11 In addition, the level of neutropenia was slightly higher than that found by Nogueira et al, at 55.1% versus 48.0%.25 The appearance of these conditions led to dose modifications for RBV (18.4%) and IFN (6.7%), or dose delays (33.7%) there-of, consistent with findings in the literature.28-32 In this study SVR rate varied according to disease stage, treatment conditions, and medication administered, with better results seen in patients without fibrosis, in those that were treatment-naïve, and in those receiving peginterferon and RBV. These results were expected and are consistent with the literature, although it should be stressed that for the other scenarios treatment with peginterferon and RBV can be difficult. In previously untreated patients, the SVR rates in this study (40.8%) were slightly higher than for other studies: in a meta-analysis by Kjaergard et al32 SVR was 37%, and in a study by Andriulli et al34 the rate was 39.6%. However, in patients who had relapsed following previous treatment, the SVR rate was similar to other studies35, 36 supporting our conclusion that this is an ideal subgroup of patients for re-treatment.37
Cirrhotic patients and previously treated patients may have lower rates of SVR than patients with low levels of fibrosis and those who are treatment-naïve.34 Our findings show that, despite being effective and well tolerated, dual therapy results in lower rates of SVR in cirrhotic patients compared with patients without fibrosis. In addition, the viral load found in this study was much higher than supported by McHutchison11 as a good predictor of SVR, which may have contributed to low response rate presented in this study.
Despite differences in SVR rates with different types of IFNs, Acras et al38 have shown that the SVR rates with the various interferons distributed by the Brazilian Government, regardless of their origin, are similar to those found globally for treatment-naïve patients. Nevertheless, the pace of evaluation and uptake of new medications has been slow. So far, only first generation IPs are available through the National Health System: boceprevir or tela previr are used in triple therapy with peginterferon and RBV in patients with genotype 1 virus and show substantially better SVR rates compared with dual therapy with peginterferon and RBV, at 75% versus 44%39 and 67% versus 41%,12 respectively.
Importantly, genotype 2, regarded as one of the most favorable responders to interferon was not evaluated due to no prevalence in the research study region. This genotype is more prevalent in European countries such as Sweden, Italy and Latin American countries such as Argentina and Venezuela,40, 41 places where such drugs may be more effective. In addition, the patients studied were not tested for IL28B rs12979860 polymorphism, SVR predictor. Like interferon, IL-28 cytokines are induced by infection and play antiviral role: CC patients for the IL28B polymorphism are three times more likely to eliminate the HCV compared to CT and TT genotype patients.42 The relationship between genotypes and RVR and SVR with this suggested that, genetic evaluation IL28B can be useful for predicting the response in patients treated with PEG-IFN and RBV.42 According to these authors, the PEG-IFN and ribavirin combination is useful, among other cases, in HCV-2 to obtain a RVR patients in the absence of cirrhosis. Since the direct antiviral action (DAAs), potentially active against this genotype, should be explored to increase without RVR patients whose response SVR rates remain unsatisfactory with the combination of PEG-IFN / ribavirin. The use of such a combination may be even more effective in cases where the viral load is below 600.00 IU/ml.11
The DAAs, these drugs are often indicated for patients with advanced fibrosis (Figure 3 and Figure 4) or non-responders to previous treatment 4. In Brazil, simeprevir, sofosbuvir, and daclatasvir have recently become available from the National Health System; however, due to access issues, the transition to these drugs has been gradual, with traditional therapies remaining the treatment of choice in less severe cases. Sofosbuvir and daclatasvir are associated with increased SVR rates, favorable tolerability, powerful antiviral activity, broad genotypic coverage, and shorter treatment duration.13, 43
Conclusion
This study shows that treatment of Hepatitis C disease with dual therapy results in a low response rate high likelihood of complications. Side-effects are common in response to treatment, with at least five clinical reactions occurring per patient, including major hematologic complications such as anemia and neutropenia. Pharmacotherapy needed adjustments, requiring rearrangement in IFN and RBV doses and the temporary suspension of treatment. These conditions raise the need for multidisciplinary care.
The future treatment of Hepatitis C looks promising, with SVR likely to reach close to 100% and a reduction in side effects associated with treatment; theremore, treatment with PEG-IFN and RBV is not justified in certain cases, but they are reasonably applied in patients with genetic characteristics and stage of the disease the more favorable RVS and in countries where patients have restricted access to new drugs.
Study limitations and future prospects
The study has some limitations, such as selection bias. As this was not a prospective, randomized, controlled trial, but a retrospective analysis of the medical records of patients who received drugs to treat Hepatitis C, there is the likelihood that information will have been lost, which will affect the results.
We would like to emphasize that the use of boceprevir and telaprevir was not investigated in this study because data were not available; this was due to the cut-off date of the study. More recent therapies need to be investigated.
Conflicts of interest. The authors declare no conflicts of interest.
Referencias
- World Health Organization (WHO). Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva: WHO; 2014. (acesso em: 2014 jun 20) Disponível em http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/.
- Péret LA. Manifestações dermatológicas durante o tratamento para hepatite C crônica com interferon alfa peguilado e ribavirina. [Dissertação] Minas Gerais, Faculdade de Medicina da Universidade Federal de Minas Gerais, 2006.
- Mohammad RA, Bulloch MN, Chan J, Deming P, Love B, Smith L, Dong BJ. Provision of clinical pharmacist services for individuals with chronic hepatitis C viral infection. Pharmacotherapy 2014; 34: 1341-1354.
- Ministério da Saúde (Brasil), Secretaria de Vigilância em Saúde, Departamento de DST, Aids e Hepatites Virais. Protocolo Clínico e Diretrizes Terapêuticas para Hepatite Viral C e Coinfecções: manejo do paciente infectado cronicamente pelo genótipo1 do HCV e fibrose avançada – Suplemento 1. Brasília: Ministério da Saúde; 2013. 24p.
- McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med 1998; 339: 1485-1492.
- Bodenheimer HC Jr., Lindsay KL, Davis GL, Lewis JH, Thung SN, Seeff LB. Tolerance and efficacy of oral ribavirin treatment of chronic hepatitis C: A Multicenter Trial. Hepatology 1997; 26: 473-477.
- Brok J, Gluud LL, Gluud C. Ribavirin monotherapy for chronic hepatitis C. Cochrane Database Syst Rev 2005; 4: CD005527.
- Hofmann WP, Herrmann E, Sarrazin C, Zeuzem S. Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int 2008; 28: 1332-1343.
- Reddy KR, Wright TL, Pockros PJ, Shiffman M, Reindollar R, Fried MW, Purdum PP, Jensen D, Smith C, Lee WM, Boyer TD, Lin A, Pedder S, De Pamphilis J. Efficacy and safety of pegylated (40-kd) interferona-2a compared with interferona-2a in non-cirrhotic patients with chronic hepatitis C. Hepatology 2011; 33: 433-438.
- Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, O’Grady J, Reichen J, Diago M, Lin A, , Brunda MJ. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med 2000; 343: 1666-1672.
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, Nyberg LM. Lee WM, Ghalib RH, Schiff ER, Galati JS, Bacon BR, Davis MN, Mukhopadhyay P, Koury K, Noviello S, Pedicone LD, Brass CA, Albrecht JK, Sulkowski MS. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009; 361: 580-593.
- Asselah T. Daclatasvir plus sofosbuvir for HCV infection: An oral combination therapy with high antiviral efficacy. J Hepatol 2014; 61: 435-438.
- Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Tarek H, Jacobson I, Lawtz MD, Lok AS, Hinestrosa F, Thuluvath PJ, Schwartz H, Nelson DR, Everson GT, Eley T, Wind-Rotolo M, Huang SP, Gao M, Hernandez D, McPhee F, Sherman D, Hindes R, Symonds W, Pasquinelli C, Grasela DM. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014; 370: 211-221.
- Ministério da Saúde (Brasil), Secretaria de Vigilância em Saúde, Departamento de DST, Aids e Hepatites Virais. Relatório técnico do estudo de prevalência de base populacional das infecções pelos vírus das hepatites A, B e C nas capitais do Brasil: dados preliminares. Recife: Ministério da Saúde; 2010. 295 p.
- García TJ, Schmidt Lara PH, Morimoto TP, Higasiaraguti M, Perejão AM, Ayub MA. Efeitos colaterais do tratamento da hepatite C no polo aplicador do ABC. Ver Assoc Med Bras 2012; 58: 543-549.
- Martins T, Narciso-Schiavon JL, Schiavon LL. Epidemiologia da infecção pelo vírus da hepatite C. Rev Assoc Med Bras 2010; 57: 107-112.
- Mangia A, Minerva N, Bacca D, Cozzolongo R, Ricci GL, Carretta V, Vinelli F, Scotto G, MontaltoG,Romano M, Cristofaro G, Mottola L, Spirito F, Andriulli A. Individualized treatment duration for hepatitis C genotype 1 patients: a randomized controlled trial. Hepatology 2008; 47: 43-50.
- Smith JP, Dong MH, Kaunitz JD. Evaluation of a pharmacist managed hepatitis C care clinic. Am J Health Syst Pharm 2007; 64: 632-636.
- Manos MM, Ho CK, Murphy RC, Shvachko VA. Physical, social, and psychological consequences of treatment for hepatitis C. Patient 2013; 6: 23-34.
- Borroni G, Cazzaniga M, Andreoletti M, Ceriani R, Guerzoni P, Omazzi B, Pich MG, Prada A, Spinzi G, Terreni N, Salerno F. Low glomerular filtration rate is a risk factor for ribavirin-associated anemia in old patients with chronic hepatitis C. J Viral Hepat 2013; 20: 90-95.
- TuralaC, Planas R. Uso clínico de telaprevir: reglas de parada, predicción de la respuesta, duración de la terapia y manejo de los efectos adversos. Enferm Infecc Microbiol Clin 2013; 31: 19-25.
- Schiff E. The alcoholic patient with hepatitis C virus infection. American Journal of Medicine 1999; 107: 95-99.
- Blatt CR, Bernardo NLMC, Rosa JA, Bagatini F, Alexandre RF, Balbinotto Neto G, Farias USMR. An estimate of the cost of hepatitis C treatment for the Brazilian health system. Value in Health Regional Issues 2012; 1: 129-135.
- McGowan CE, Fried MW. Barriers to hepatitis C treatment. Li-ver Int 2012; 32:151-156.
- Nogueira JBC, Sena LCS, Quintans JSS, Almeida JR, Franca AV, Júnior LJ. Side effects of the therapy with peginterferon and ribavirin in chronic hepatitis C: A Small Audit. J Pharm Pract 2012; 25: 85-88.
- Haynes RB. Determinants of compliance: The disease and the mechanics of treatment. Baltimore MD, Johns Hopkins University Press, 1979.
- McGowan CE, Fried MW. Barriers to hepatitis C treatment. Li-ver Int 2012; 32:151-156.
- García TJ, Schmidt Lara PH, Morimoto TP, Higasiaraguti M, Perejão AM, Ayub MA. Efeitos colaterais do tratamento da hepatite C no polo aplicador do ABC. Ver Assoc Med Bras 2012; 58: 543-549.
- Bagheri H, Fouladi A, Barange K, Lapeyre-Mestre M, Payen JL, Montastruc JL, Vinel JP. Follow-up of adverse drug reactions from peginterferon alfa-2b-ribavirin therapy. Pharmacotherapy 2004; 24:1546-1553.
- Fontanges T, Beorchia S, Douvin C, Delassalle P, Combis JM, Hanslik B, Jacques JP, Filoche B, Desmorat H, Chandelier C, Ouzan D. Safety and efficacy of combination therapy with peginterferon alfa-2a (40kD) and ribavirin in the outpatient setting: prospective analysis of 197 patients with chronic hepatitis C viral infection. Gastroenterol Clin Biol 2007; 31: 566-572.
- Mariño EL, Álvarez-Rubio L, Miró S, Modamio P, Baños F, Lastra, CF, Alberdi-Leniz A. Pharmacist intervention in treatment of patients with genotype 1 chronic hepatitis C. J Manag Care Pharm 2009; 15:147-150.
- Kjaergard LL, Krogsgaard K, Gluud C. Ribavirin with or without alfa interferon for chronic hepatitis C [update of: Cochrane Database Syst Rev 2002; (1)]. Cochrane Database Syst Rev 2002; 2: CD002234.
- Ogawa E,Furusyo N, Kajiwara E, Takahashi K, Nomura H, Tanabe Y, Satoh T, Maruyama T, Nakamuta M, Kotoh K, Azuma K, Dohmen K, Shimoda S, Hayashi J. Evaluation of the adverse effect of premature discontinuation of pegylated interferon a-2b and ribavirin treatment for chronic hepatitis C virus infection: Results from Kyushu University Liver Disease Study. J Gastroenterol and Hepatol 2012; 27: 1233-1240.
- Andriulli A, Di Marco V, Margaglione M, Ippolito AM, Fattovich G, Smedile A, Valvano MR, Calvaruso V, Gioffreda D, Milella M, Morisco F, Felder M, Brancaccio G, Fasano M, Gatti P, Tundo P, Barone M, Cozzolongo R, Angelico M, D’Andrea G, Andriulli N, Abate ML, Mazzella G, Gaeta GB, Craxi A, Santantonio T. Identification of naïve HCV-1 patients with chronic hepatitis who may benefit from dual therapy with peginterferon and ribavirin. Journal of Hepatology 2014; 60: 16-21.
- Gonçales Jr FL, Vigani A, Gonçales N, Barone AA, Araújo E, Focaccia R, Oliveira U, Coelho HS, Paixao J, Perez R, Lobato C, Weirich J, Rosa H, Borges A, Vila R, Corrêa-Giannella ML, Ferraz ML. Weight-based combination therapy with peginterferon alfa-2b and ribavirin for naïve, relapser and non-responder patients with chronic hepatitis C. Braz J Infect Dis 2006; 10: 311-316.
- Shiffman ML. Management of patients with chronic hepatitis C virus infection and previous nonresponse. Rev Gastroenterol Di-sord 2004; 4: 22-30.
- Bruno S, Shiffman ML, Roberts SK, Gane EJ, Messinger D, Hadziyannis SJ, et al. Efficacy and safety of peginterferon alfa-2a (40kd) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology 2010; 51: 38.
- Acras RN, Pedroso MLA, Caum LC, Pisani J, Amarante HMBS, Carmes ER. A taxa de resposta sustentada da hepatite C crônica ao tratamento com os diversos interferons-alfa e ribavirinas distribuídos pelo governo brasileiro é semelhante à da literatura mundial. Arq. Gastroenterol 2004; 41: 3-9.
- Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky V, Moroz L, Craxi A, Peeters M, Lenz O, Ouwerkerk-Mahadevan S, De La Rosa G, Kalmeijer R, Scott J, Sinha R, Beumont-Mauviel M. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. The Lancet 2014; 384: 403-413.
- Kershenobich D, Razavi HA, Sanchez-Avila JF, Bessone F, Coelho HS, Dagher L, Gonçales FL, Quiroz JF, Rodriguez-Perez F, Rosado B, Wallace C, Negro F, Silva M. Trends and projections of hepatitis C virus epidemiology in Latin America. Liver Int 2011; 31: 18-29.
- Thomas DI, Thio CI, Martin Mp, Qi Y, Ge D. Genetic variation in IL28b and spontaneous clearance of hepatitis C virus. Nature 2009; 461: 798-801.
- Mangia A, Mottola L. What’s new in HCV genotype 2 treatment. Liver International 2012; 135-140.
- Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcelin P, Muir AJ, Ferenci P, Flisiak R, Geroge J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda A, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364: 2405-2416.
Correspondencia: Marcos Cardoso Rios
Laboratory of Research and Teaching in Social Pharmacy, Department of Physiology, Federal University of Sergipe
Av. Marechal Rondon, s/n, Jardim Rosa Elze, 49100-000, São Cristóvão, Sergipe, Brazil – Phone/Fax: + 5507988084488.
Correo electrónico: mcrios_farma@yahoo.com.br
Acta Gastroenterol Latinoam 2017;47(1): 14-22
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE