Blanca Medrano-Engay,1, 6 Pilar Irún,2 Marcio Andrade-Campos,1, 3 Fernando Medina- Monroy,4 Alberto Almeida,1, 7 Miguel Pocoví,5 Pilar Giraldo1, 3, 6
1 Unidad de Investigación Trasnacional, Hospital Universitario Miguel Servet. Instituto de Investigación Sanitaria Aragón (IIS).
2 Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), ISCIII.
3 Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER), ISCIII. Zaragoza, Spain.
4 Unidad de Gastroenterología, Nutrición y Endoscopia Pediátrica (UGANEP). Bucaramanga, Colombia.
5 Departamento de Bioquímica y Biología Molecular y Celular, Universidad de Zaragoza.
6 Fundación Española para el Estudio y Terapéutica de la Enfermedad de Gaucher (FEETEG).
7 Universidad San Jorge. Zaragoza, Spain.
Acta Gastroenterol Latinoam 2019;49(1):54-64
Recibido: 26/03/2018 / Aprobado: 13/07/2018 / Publicado en www.actagastro.org el 18/03/2019
Summary
The iminosugar miglustat, is a treatment for some lysosomal storage disorders that induces gastrointestinal side effects. A retrospective study that evaluates two different tests to assess lactose intolerance was done with the aim to determine whether lactose intolerance is responsible for gastrointestinal symptoms in patients with type 1 Gaucher’s disease. Forty patients with Gaucher’s disease treated or not with miglustat and twenty controls. For that they ingested 50 g lactose, followed by a hydrogen methane breath test. Genotyping and sequencing of a single nucleotide polymorphism (SNP) rs4988235, (CC genotype for lactase non-persistence; TC/TT for lactase persistence) were tested on them. Gastrointestinal symptoms were present in 47.6% (19/40) of patients, 35% (14/40) of whom had a positive hydrogen methane breath test; of these 14 patients, 12 patients were CC-positive and 2 patients were TC-positive. The number of CC alleles was significantly greater in Gaucher’s disease patients compared to controls (62.5% vs. 43.8%). Of the patients who had gastrointestinal symptoms on miglustat treatment, 60.0% had the CC genotype. Conclusion. Hydrogen methane breath test and genotyping for SNPs could be used indistinctly to diagnose lactase deficiency. We have observed that not all gastrointestinal symptoms induced by miglustat in Gaucher’s disease are justified by lactase deficiency.
Key words. Breath test, Gaucher disease, lactose intolerance, polymorphism single nucleotide.
Prueba de aliento y genotipado para la intolerancia a la lactosa en pacientes con enfermedad de Gaucher tratados con miglustat
Resumen
El iminoazúcar miglustat, medicamento empleado para tratar algunas enfermedades de depósito lisosomal, induce efectos adversos gastrointestinales. Se ha llevado a cabo un estudio retrospectivo que compara dos pruebas de diagnóstico diferentes para evaluar la intolerancia a la lactosa con el objetivo de determinar si esta intolerancia es responsable de los síntomas adversos gastrointestinales en pacientes con enfermedad de Gaucher tipo 1. Cuarenta pacientes con enfermedad de Gaucher tratados o no con miglustat y veinte controles. Para ello ingirieron 50 g de lactosa, y a continuación efectuaron la prueba de aliento de hidrógeno y metano. Además, se les realizó genotipificación y secuenciación de un polimorfismo de un solo nucleótido (SNP) rs4988235, (CC: no persistencia de lactasa, TC / TT: persistencia de lactasa). Los síntomas gastrointestinales estaban presentes en el 47,6% (19/40) de los pacientes, el 35% (14/40) de los cuales tenían una prueba positiva de aliento de hidrógeno y metano; de estos 14 pacientes, 12 pacientes fueron CC positivos y 2 pacientes fueron TC positivos. El número de alelos CC fue significativamente mayor en los pacientes con enfermedad de Gaucher en comparación con los controles (62,5% frente a 43,8%). De los pacientes que tenían síntomas gastrointestinales en el tratamiento con miglustat, 60,0% tenían el genotipo CC. Conclusión. La prueba de aliento de hidrógeno y el genotipado para SNP podrían usarse indistintamente para diagnosticar la deficiencia de lactasa. Hemos observado que no todos los síntomas gastrointestinales inducidos por miglustat en la enfermedad de Gaucher se justifican por la deficiencia de lactasa.
Palabras claves. Test de aliento, enfermedad de Gaucher, intolerancia a la lactosa, polimorfismo de un solo nucleótido.
Abbreviations
SNP: single nucleotide polymorphism.
CC: genotype for lactase non-persistence.
TC/TT: for lactase persistence.
GD1: type 1 Gaucher’s disease.
CEIC: Ethical Committee for Clinical Research.
ppm: parts per million.
PCR: polymerase chain reaction.
PPV: positive predictive value.
NPV: negative predictive value.
LBT: lactose hydrogen methane breathe test.
Type 1 Gaucher’s disease (GD1) (OMIM 230800) is an inborn autosomal-recessive disorder of lysosomal metabolism characterized by the accumulation of glucocerebroside in different organs and systems. It is a consequence of the deficiency in the activity of the enzyme glucocerebrosidase (acid b-glucosidase).1
The actual therapy of GD1 is based in the periodic intravenous administration of enzyme replacement therapy used widely since 1990. Other strategy of therapy was introduced in 2002 in EU with an oral inhibitor of substrate accumulation (miglustat) that induce a reversible blocking of the enzyme glucosylceramide synthase that catalyses the rate-limiting stage in the glycosphingolipid biosynthesis pathway.2
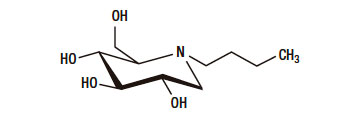
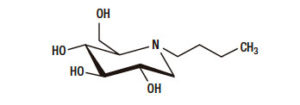
Miglustat is a synthetic iminosugar, analogue of D-glucose (Figure 1), is a derivative of an alpha-glucosidase inhibitor (N-butyl-Deoxynojirimycin). The alpha-glucosidase inhibitors act like competitive inhibitors of the carbohydrates in need to digest by the enzymes: specifically alpha-glucosidase enzymes in the brush border of the small intestines. The membrane-bound intestinal alpha-glucosidases hydrolyze oligosaccharides, trisaccharides, and disaccharides to glucose and other monosaccharides in the small intestine. Due to this, in the colon, bacteria will digest the non-digested complex carbohydrates and induce side effects such as gastrointestinal bloating and diarrhoea.3
Figure 1. Chemical formulation of N-butyl-Deoxynojirimycin.
The other derivatives of alpha-glucosidase N-hydroxyethyl-deoxynojirimycin are oral anti diabetic drugs that work by preventing the digestion of carbohydrates.
Gastrointestinal side effects such as diarrhoea, flatulence and abdominal pain/discomfort have been described in more than 60% of the patients with Gaucher’s disease who are treated with miglustat during clinical trials and in real-world clinical practice settings. These adverse events are generally mild or moderate in severity and occurring moistly during the initial weeks of therapy, however, they constitute the major cause of discontinuation.4 Some studies have shown in vitro that miglustat inhibits intestinal disaccharidases such as sucrose, maltase, and also lactase.5 Furthermore, also in vivo, miglustat shows the ability to inhibits the digestion of maltose, sucrose, starch and the final hydrolysis and absorption of oligosaccharides and disaccharides.6
Functional gastrointestinal disorders (GI) are very common, with an incidence around 40% among general population.7 Up to 30% of those cases could be justified by an intolerance to lactose in the meridional countries.8 Lactase non-persistence phenotype is linked to an inherited single polymorphism trait, in the Caucasian population, is irregularly distributed, ranging from 3 to 70% depending to the geographical areas in Europe. Higher incidence reaching up to 100% had founded in other populations such Asians.9, 10
Nevertheless, secondary lactase deficiency is an acquired and reversible form that may occurs in diseases affecting large areas of the small intestinal mucosa and can be observed associated to several infections as tropical sprue, infectious enteritis, small bowel bacterial overgrowth or inflammatory chronic diseases as bowel disease, celiac or different situations as, short bowel syndrome, radiation enteritis, gastrointestinal surgery, drugs this situation is more common in countries with a high prevalence of infection by intestinal parasites.9, 11, 12
Lactose malabsorption is defined as an inefficient lactose digestion due to lactase non-persistence or due to intestinal disease, such as inflammation.13
Lactose malabsorption exists in three different forms: congenital, primary, and secondary. Congenital lactase deficiency is an extremely rare autosomal recessive, life-long gastrointestinal disorder, leading to watery diarrhoea from the first exposure to breast milk in infants.14-16 Primary lactose malabsorption, also known as adult-type hypolactasia or lactase non-persistence, is the most common phenotype found in humans and results in a decline in lactase enzymatic activity in the small intestine. A single nucleotide polymorphism (TC-13910) 14 kb upstream from the lactase gene locus is associated with adult-type hypolactasia.14, 15, 17
The detection of the lactase gene locus -13910 C > T polymorphism, with CC genotype for lactase non-persistence and TC and TT genotypes for lactase persistence, has been shown previously to be as reliable as the hydrogen methane breath test for predicting lactose malabsorption in European populations.18-20
The prospective study presented here try to deep in the most common adverse effect induced by miglustat therapy and evaluate the influence of lactose intolerance and lactose malabsorption, determined by non-invasive tests (hydrogen methane breath test and genotyping for the single nucleotide polymorphism rs4988235) in the development of gastrointestinal symptoms in patients with Gaucher’s disease exposed or not to therapy with miglustat.
Patients and methods
Ethical approval
The study was conducted according to a study design approved by the Ethical Committee for Clinical Research, Aragón (CEIC Aragón), Spain. The research study was conducted according to the Declaration of Helsinki. All subjects, who participated in the study, agreed to participate in the study and signed informed consents.
Subjects studied
A prospective study was designed and conducted at the Gaucher’s Unit of the Translational Research Unit, Miguel Servet University Hospital, IIS Aragon, Spain, between January 2013 and December 2014. The studied subjects included a group comprised by 40 patients with Gaucher’s disease, selected from the Spanish Registry of Gaucher’s Disease: 17 (42.5%) male patients and 23 (57.5%) female patients, median age 45 years (range: 14- 76), and a group of 20 healthy controls: 7 (35%) male subjects and 13 (65%) female subjects, median age 40.5 years (range: 23-56).
Prior to the start of the study, a detailed clinical history was recorded for each patient in both groups, including concomitant drugs administration and type of therapy for Gaucher’s disease and a history of gastrointestinal symptoms.
Hydrogen methane breath test
The hydrogen methane breath test was performed following a 6-hour overnight fast. Baseline breath concentrations of hydrogen and methane were measured. Oral lactose was given at a dose of 50 g dissolved in 300 mL of water. The amount of exhaled hydrogen and methane was measured at 25, 50, 75, 100, 125, 150, and 175 minutes following lactose ingestion. The breath measurements of hydrogen and methane were expressed in parts per million (ppm).
Prior to breath testing, patients were asked to report clinical symptoms and to avoid eating, smoking, and physical efforts. Additionally, individuals who underwent colonoscopy or were taking any antibiotics in the fortnight before the test were excluded from the study.20
The breath test result was considered positive if the hydrogen (H2) peak was > 20 ppm above the baseline value and/or the methane (CH4) peak was > 12 ppm above the baseline value in at least two sequential measurements. Patients were classified as lactose malabsorbers if a breath hydrogen increase > 20 ppm and/or a breath methane increase > 12 ppm above the baseline value were observed within 75-175 minutes following the lactose ingestion. If lactose malabsorption was accompanied by gastrointestinal symptoms, patients were classified as lactose intolerant. Lactose-dependent small intestinal bacterial overgrowth was considered if there was an increase of > 20 ppm in breath hydrogen and/or in the breath methane peak of > 12 ppm above, the baseline was observed up to 75 minutes following lactose ingestion.
Gas chromatography was used to measure the hydrogen and methane concentrations in exhaled breath using the QuinTron Breath Tracker analyser (QuinTron Instrument Co., Milwaukee, WI, USA). The analysis was performed centrally by ISOMED according manufacturer protocol. ISOMED PHARMA Laboratories, Spain.
Molecular analysis
Peripheral venous blood samples were obtained from each study participant and DNA was isolated using the Nucleon BACC3 kit (Amersham Biosciences, UK). The genotyping procedure involved a polymerase chain reaction (PCR) and amplification and sequencing on an ABI3500XL capillary genetic analyser (Applied Biosystems). For PCR amplification, the forward used primer was: 5´TTCATGGAGGATTACAGTGCGACAG3´ and the reverse used primer were: 5´AGACGACCTTACATCAAACCTATTA3´.
The PCR mixture contained 0.2 μM primers, 1× PCR buffer, 1.5 mM MgCl2, 0.17 mM each dNTP, 0.5 U Taq polymerase (Bio-line, UK) and 100 ng of DNA in a total volume of 15 μl. Cycling conditions were 94°C for 2 min, followed by 35 cycles of 25 sec at 94°C, 20 sec at 55°C and 40 sec at 72°C, and 7 min at 72°C as a final extension step. The PCR product was purified using ExoSAP-IT (Affymetrix) and sequenced. All products were separated on the 3500XL Genetic Analyser (Applied Biosystems) and analysed with Variant Reporter Software v1.1.
With these procedures we have analysed in every subject the variants TG 13915, CT 13910 and CG 13907 in the lactase gene.
Statistics
Statistical analysis was performed with SPSS18.0 software. Descriptive statistics and nonparametric statistical (Pearson test, Fisher test, U-Mann-Whitney) and correlation analyses were performed. Positive predictive value (PPV), negative predictive value (NPV), specificity and sensitivity were calculated between results from the hydrogen methane breath test and molecular testing. Statistical agreement was calculated using Cohen’s kappa statistics (K).21 Differences were considered statistically significant when p < 0.05.
Results
Concordance between the hydrogen methane breath test and molecular testing
In Figure 2 there is detailed the results of Lactose hydrogen methane Breath Test (LBT) and the genotyping assays in every group. Only 14/40 (35%) Gaucher’s disease patients and 5/20 (25%) of controls had a LBT positive, while 26/40 (65%) and 15/20 (75%) respectively showed a negative hydrogen methane breath test. In Table 1 is detailed the general characteristics of patients and controls.
Figure 2. Results of Lactose Breath Test (LBT) and genotyping assays.
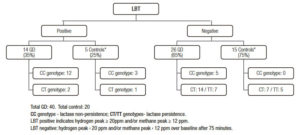
Table 1. General characteristics of both groups: Gaucher’s disease (GD) and control.
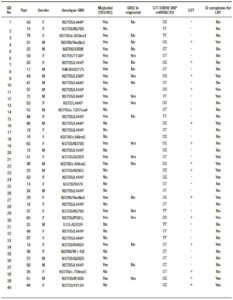
Regarding the genetic study, in Table 2 is showing the distribution of polymorphism CT-13910 in patients and control group. Genotyping revealed that the lactase non-persistence genotype (CC-positive) were present in 17/40 (42.5%) patients. Of the CC-negative patients (23/40, 57.5%), 7/40 (17.5%) were TT homozygous and 16/40 (40.0%) carried the heterozygous genotype TC. The others polymorphisms (TG-13915 and CG-13907) were analysed in all subjects and they are not expressed.
The comparison between the hydrogen methane breath tests and the molecular tests indicates that from the 17 Gaucher’s disease patients carrying the CC genotype, 12 had a positive hydrogen methane breath test (71%) (p = 0.0001) (Figure 3). Respect to the control group, the genotyping (16/20 analysed) revealed a lower presence of the lactase non-persistence genotype (3/16, 18.7%) and all of the CC carriers also presented a positive hydrogen methane breath test results (p = 0.007) Figure 3. In the CC-negative carriers controls (13/16, 81.3%), 5 (31.3%) were TT homozygous and 8 (50.0%) carried the heterozygous genotype TC (Table 2).
Figure 3. Concordance between Lactose Breath Test (LBT) and CC, CT/TT genotypes.
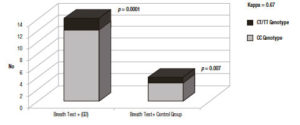
Table 2. Distribution CT- 13910 polymorphism in Gaucher’s disease (GD) and control groups.
Discordance between the hydrogen methane breath test and molecular testing
The Table 3 shows the genetic profile and the hydrogen methane breath test distribution in the Gaucher’s disease patients and control groups. Globally, in 5/38 (13.2%) CC-positive subjects, the hydrogen methane breath test was negative and 3/18 (16.7%) CC negative subjects tested were positive in the hydrogen methane breath test, demonstrating a strong correlation between both non-invasive assessments (p = 0.0001).
Table 3. Discordance between lactose breath test (LBT) and genotyping in both groups.
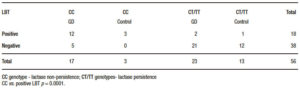
The Cohen’s kappa statistical analysis showed a statistical agreement (0.67) between the two methods, hydrogen methane breath test and genotyping.21 When compared with the genotyping, the sensitivity of the hydrogen methane breath test was 75% and 92% the specificity, while the negative and positive predictive values (NPV and PPV) were 87%, and 83% respectively. In subjects with at least one T allele, the presence of a negative hydrogen methane breath test indicates a lactase persistence phenotype. While in subjects non-carrying a T allele, the positivity of the hydrogen methane breath test identified the subjects with the lactase non-persistent phenotype. Non-agreement between both techniques (false positive and false negatives) could be related with other causes like secondary lactase deficiency.
The clinical records of the two patients with a negative genetic test but a positive hydrogen methane breath test, was further investigated (to discharge secondary causes of lactose intolerance). One 26-year-old woman with TC genotype), had no reported gastrointestinal symptoms in spite of the positive hydrogen methane breath test. The other case, an 18-year-old woman with TC phenotype showed a 60 and 72 ppm hydrogen methane basal levels, which were maintained in four subsequent measurements. It was assumed that this patient had malabsorption due to intestinal bacterial overgrowth, as she also reported symptoms of bloating and abdominal pain.
The clinical records of the five Gaucher’s disease patients who had a positive molecular test but a negative hydrogen methane breath test were investigated. Two patients probably corresponding to have intestinal bacterial overgrowth, and one had infection with Helicobacter pylori. In three other patients, no associated gastrointestinal disease could be found, but in two of these cases lactase-persistence could have been mediated by other no detected genetic polymorphisms. One patient, a 16-year-old man with a CC genotype, may have had lack of genotype expression prior to adulthood, as has been previously reported.22
From the control group a 56-year-old woman with a TC genotype (lactase persistence phenotype), who showed a positive hydrogen methane breath test, also reported symptoms of irritable bowel syndrome that can be the cause of the secondary lactase deficiency.
Gastrointestinal symptoms
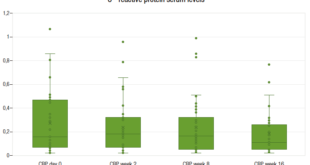
In 26 GD patients with negative hydrogen methane breath test 3 (11.5%) were developed gastrointestinal symptoms during the procedure. By contrast, in 14 GD patients with positive hydrogen methane breath test other 3 (21.4%) have not any gastrointestinal symptoms during the test. In the control group, the percentage of subjects with negative hydrogen methane breath and gastrointestinal manifestations during the procedure was higher 26.6% but without statistical significance, only one subject in the positive breath test group has not any gastrointestinal symptoms (Figure 4).
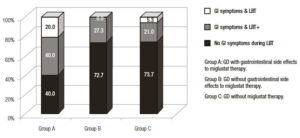
Regarding the subgroup of Gaucher’s disease patients exposed to miglustat who reported gastrointestinal symptoms secondary to therapy, 10/21 (47.6%) (A subgroup); the results of the hydrogen methane breath test are detailed in Figure 4. Of them, only 4 patients (40.0%), fulfilled the diagnosis of lactose intolerance (breath test positive and development of symptoms during the test) as described in material and methods and 2 (20.0%) had a negative hydrogen methane breath test and gastrointestinal symptoms. The B subgroup, 11/21 (52.4%) Gaucher’s disease patients who had intake miglustat therapy without reporting gastrointestinal symptoms secondary to therapy, 8 (72.7%) did not have gastrointestinal symptoms during the hydrogen methane breath test, in spite of the fact that in 4 of them the hydrogen methane breath test was positive Figure 5. In the group of patients never exposed to miglustat therapy, C subgroup, 5/19 (26.3%) had gastrointestinal symptoms during the hydrogen methane breath test and positive hydrogen methane breath test, except one patient with a negative hydrogen methane breath test and gastrointestinal symptoms (Figure 5).
Figure 4. Percentage of Lactose Breath Test (LBT) and Gastrointestinal (GI) symptoms in Gaucher’s disease (GD) group and control group.
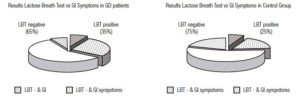
Figure 5. Gastrointestinal (GI) symptoms related to lactose breath test (LBT) in different groups of Gaucher’s disease (GD) patients exposed or no to miglustat.
Concerning the results obtained in the alimentary habits, medicines intake and gastrointestinal rhythm survey, we have observed that 10/40 patients considered their-selves as poor tolerance to milk and avoid their ingestion, of them six had a positive hydrogen methane breath test (60%) and 4 were CC genotype.
Discussion
The purpose of this prospective study was to determine whether lactose intolerance was responsible for gastrointestinal symptoms in patients with Gaucher’s disease and to evaluate two diagnostic tests for this association. Genotyping and sequencing of a single nucleotide polymorphism rs4988235, were performed using polymerase chain reaction (CC genotype for lactase non-persistence and the TC/TT genotypes for lactase-persistence) and the hydrogen methane breath test were compared.
In this study, around a half of patients under miglustat therapy had reported mild to moderate gastrointestinal symptoms and the third part had a lactose positive hydrogen methane breath test. In Spain, the incidence of gastrointestinal symptoms in patients with Gaucher’s disease treated with miglustat is lower than expected, possibly due to the Mediterranean diet and the low carbohydrate dietary intake recommended during the first weeks of therapy.23
Concerning the concordance between the positivity of the lactose hydrogen methane breath test and CC genotype, a strong correlation was founded; in fact, the 83.3% of subjects with a positive lactose breath test were CC-positive. The total number of C alleles was significantly greater in Gaucher’s disease patients compared with controls (62.5% vs. 43.8%). Of the patients who had gastrointestinal symptoms on miglustat treatment, 60.0% had the CC genotype. These findings in line with some previous studies as the ones performed by Krawczyk in European population.20 However, the value obtained in the Cohen’s kappa statistics (0.67) for agreement assessment was slightly inferior to that reported in previous studies18, 20 but superior to the published by Enko.11
In the present study, from the Gaucher’s disease patients with a CC-negative genotype (n = 23), the majority 21 (91.3%) had a negative breath test, and only two (8.7%) had a positive breath test who may have suffered from secondary lactase deficiency or other conditions that require further clinical investigation.
Among the limitations of the hydrogen methane breath test performance and the posterior measurement were both user-dependent and dependent upon non-controlled pre-analytical factors.24, 25 Other factors as the previous use of antibiotics.26 The colonic pH and the orocecal transit time can influence in the accuracy of the test and cause false negative results.27-30 However, in our study, subjects who had completed antibiotic therapy were excluded for at least 4 weeks from the breath test, because this exposition could trigger alterations of the colonic bacterial flora. Based on the followed protocol we assume that, external factors have not influenced the results of the study and the variations obtained among the different polymorphisms needs to be assumed considering that the molecular analysis of SNPs may not be able to explain all the variations of lactase expression, due to genetic heterogeneity in the regulation of this expression as has been previously reported,31 in our study we have analysed the TG-13915 and CG-13907 polymorphism that are nearest of CT 13910 in the lactase gene following previous studies, which described them as associate variants to lactase persistence in different populations including the Caucasians.32
In some cases, it is may be difficult to interpret adequately the clinical manifestations, for example the symptoms of lactose intolerance may have been exaggerated, while some individuals with lactase non-persistence may be well-tolerate up to 12 g of oral lactose,33 although many individuals may now diagnose themselves as being lactose intolerant, these individuals may be lactase persistent and be suffering from irritable bowel syndrome.
An important finding of this study was that the presence of gastrointestinal symptoms induced by miglustat were not directly related with the lactose tolerance genotype, the observed differences among the cases with lactose malabsorption phenotype in Gaucher’s disease patients treated or not with miglustat and control group were not significant. In order to explain the gastrointestinal symptoms in Gaucher’s and other lysosomal patients treated with miglustat, other intestinal enzymes involved in the absorption of sucrose and maltose must be studied to perform a more complete intestinal enzyme profile. Dietary modifications such as reduced consumption of dietary sucrose, maltose and lactose have been shown to improve the gastrointestinal tolerability of miglustat and reduce the magnitude of any changes in body weight, particularly if it is initiated at or before the start of therapy. Also miglustat dose escalation at treatment initiation may also reduce gastrointestinal disturbances.34
Conclusion
Hydrogen methane breath test and/or the genotyping for SNPs may be used indistinctly to diagnose lactase deficiency because there is a high concordance in the results. In this study we have observed that not all gastrointestinal symptoms induced by miglustat in Gaucher’s disease are justified by lactase deficiency.
Acknowledgements. To patients and participants in the study for their invaluable collaboration and selfless participation. To Charleston Group. Cambridge Language for the English editing review.
Financial support. This work has been partially granted by PS 12/01219, PS15/00616 and FEETEG.
Conflict of interest. Blanca Medrano-Engay, Pilar Irún, Alberto Almeida, Miguel Pocoví and Fernando Medina-Monroy declares no conflict of interest. Pilar Giraldo has received research support, educational grants, and travel support, honoraria for consultancies and speakers fees from Genzyme, Shire HGT, Amicus, Protalix and Actelion. All fees are donated to the Spanish Gaucher Foundation for research support.
Authorship
Blanca Medrano-Engay and Pilar Giraldo: study design, data collection, data analysis and interpretation of results, draft of manuscript.
Alberto Almeida: laboratory support.
Pilar Irún, Miguel Pocoví: participation in procedure and interpretation of genetic tests, revision of manuscript.
Marcio Andrade-Campos: interpretation of results, revision of manuscript and editing English.
Fernando Medina-Monroy: revision of manuscript and editing English.
References
- Beutler E, Grabowski G. Gaucher Disease. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease 7th ed. New York: McGraw Hill; 1995. 2641- 2670.
- Elstein D, Dweck A, Attias D, Hadas-Halpern I, Zevin S, Altarescu G, Aerts JF, van Weely S, Zimran A. Oral maintenance clinical trial with miglustat for type I Gaucher disease: switch from or combination with intravenous enzyme replacement. Blood 2007; 110: 2296-2301.
- Nash RJ, Kato A, Yu CY, Fleet GW. Iminosugars as therapeutic agents: recent advances and promising trends. Future Med Chem. 2011; 3: 1513-1521.
- Mehta A, Hollak CE, Giraldo P, Hughes D, Belmatoug N, Brand M, Muller A, Schaaf B, Giorgino R, Zimran A. Miglustat therapy in type 1 Gaucher disease: clinical and safety outcomes in a multicenter retrospective cohort study. Blood Cells Mol Dis 2013; 51: 116-124.
- Amiri M, Naim HY. Miglustat-induced intestinal carbohydrate malabsorption is due to the inhibition of alpha-glucosidases, but not beta-galactosidases. J Inherit Metab Dis 2012; 35: 949-954.
- Andersson U, Butters TD, Dwek RA, Platt FM. N-butyldeoxygalactonojirimycin: a more selective inhibitor of glycosphingolipid biosynthesis than N-butyldeoxynojirimycin, in vitro and in vivo. Biochem Pharmacol 2000; 59: 821-829.
- Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil 2008; 20: 121-129.
- Camilleri M, Murray J. Harrison Principios de Medicina Interna. 17 ed. Mexico DF: McGraw-Hill Co; 2009.
- Gudmand-Høyer E, Skovbjerg H. Disaccharide digestion and maldigestion. Scand J Gastroenterol 1996; 216: 111-121.
- Sahi T. Genetics and epidemiology of adult-type hypolactasia. Scand J Gastroenterol 1994; 202: 7-20.
- Enko D, Rezanka E, Stolba R, Halwachs-Baumann G. Lactose malabsorption testing in daily clinical practice: a critical retrospective analysis and comparison of the hydrogen/methane breath test and genetic test (c/t-13910 polymorphism) results. Gastroenterol Res Pract 2014; 2014: 464382.
- Saavedra J, Perman J. Current concepts in lactose malabsorption and intolerance. Annu Rev Nutr 1989; 9: 475-502.
- Misselwitz B, Pohl D, Frühauf H, Fried M, Vavricka SR, Fox M. Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterol J 2013; 1: 151-159.
- Usai-Satta P, Scarpa M, Oppia F, Cabras F. Lactose malabsorption and intolerance: What should be the best clinical management? World J Gastrointest Pharmacol Ther 2012; 3: 29-33.
- Madry E, Fidler E, Walkowiak J. “Lactose intolerance-current state of knowledge,” Acta Scientiarum Polonorum, Technologia Alimentaria 2010; 9: 343-350.
- Kokkonen J, Enattah NS, Ylisaukko-Oja T, Komu H, Varilo T, Peltonen L, Savilahti E, Jarvela I. Mutations in the translated region of the lactase gene (LCT) underlie congenital lactase deficiency. Am J Hum Genet 2006; 78: 339-344.
- Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Järvelä I. Identification of a variant associated with adult-type hypolactasia. Nat Genet 2002; 30: 233-237.
- Pohl D, Savarino E, Hersberger M, Behlis Z, Stutz B, Goetze O, Eckardstein AV, Fried M, Tutuian R. Excellent agreement between genetic and hydrogen breath tests for lactase deficiency and the role of extended symptom assessment. Br J Nutr 2010; 104: 900-907.
- Högenauer C, Hammer HF, Mellitzer K, Renner W, Krejs GJ, Toplak H. Evaluation of a new DNA test compared with the lactose hydrogen breath test for the diagnosis of lactase non-persistence. Eur J Gastroenterol Hepatol 2005; 17: 371-376.
- Krawczyk M, Wolska M, Schwartz S, Gruenhage F, Terjung B, Portincasa P, Sauerbruch T, Lammert F. Concordance of genetic and breath tests for lactose intolerance in a tertiary referral centre. J Gastrointestin Liver Dis 2008;17: 135-139.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159-174.
- Seppo L, Tuure T, Korpela R, Jarvela I, Rasinpera H, Sahi T. Can primary hypolactasia manifest itself after the age of 20 years? A two-decade follow-up study. Scand J Gastroenterol 2008; 43: 1082-1087.
- Giraldo P, Alfonso P, Atutxa K, Fernández-Galán MA, Barez A, Franco R, Alonso D, Martin A, Latre P, Pocovi M. Real-world clinical experience with long-term miglustat maintenance therapy in type 1 Gaucher disease: the ZAGAL project. Haematologica 2009; 94: 1771-1775.
- Thompson DG, Binfield P, De Belder A, O’Brien J, Warren S, Wilson M. Extra intestinal influences on exhaled breath hydrogen measurements during the investigation of gastrointestinal disease. Gut 1985; 26: 1349-1352.
- Payne DL, Welsh JD, Claypool PL. Breath hydrogen (H2) response to carbohydrate malabsorption after exercise. J Lab Clin Med 1983; 102: 147-150.
- Gilat T, Ben Hur H, Gelman-Malachi E, Terdiman R, Peled Y. Alterations of the colonic flora and their effect on the hydrogen breath test. Gut 1978; 19: 602-605.
- Perman JA, Modler S, Olson AC. Role of pH in production of hydrogen from carbohydrates by colonic bacterial flora. Studies in vivo and in vitro. J Clin Invest 1981; 67: 643-650.
- Vogelsang H, Ferenci P, Frotz S, Meryn S, Gangl A. Acidic colonic microclimate possible reason for false negative hydrogen breath tests. Gut 1988; 29: 21-26.
- El Oufir L, Flourié B, Bruley des Varannes S, Barry JL, Cloarec D, Bornet F, Galmiche JP. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut 1996; 38: 870-877.
- Eisenmann A, Amann A, Said M, Datta B, Ledochowski M. Implementation and interpretation of hydrogen breath tests. J Breath Res 2008; 2: 046002.
- Poulter M, Hollox E, Harvey CB, Mulcare C, Peuhkuri K, Kajander K, Sarner M, Korpela R, Swallow DM. The causal element for the lactase persistence/non-persistence polymorphism is located in a 1 Mb region of linkage disequilibrium in Europeans. Ann Hum Genet 2003; 67: 298-311.
- Tishkoff SA, Reed FA, Ranciaro A, Voight BF, Babbitt CC, Silverman JS, Powell K, Mortensen HM, Hirbo JB, Osman M, Ibrahim M, Omar SA, Lema G, Nyambo TB, Ghori J, Bumpstead S, Pritchard JK, Wray GA, Deloukas P. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet 2007; 39: 31-40.
- Mattar R, de Campos Mazo DF, Carrilho FJ. Lactose intolerance: diagnosis, genetic, and clinical factors. Clin Exp Gastroenterol 2012; 5: 113-121.
- Belmatoug N, Burlina A, Giraldo P, Hendriksz CJ, Kuter DJ, Mengel E, Pastores GM. Gastrointestinal disturbances and their management in miglustat-treated patients. J Inherit Metab Dis 2011; 34: 991-1001.
Correspondencia: María Soledad Muñoz
Paseo Isabel La Católica 1-3 (50009). Traslational Research Unit.Miguel Servet University Hospital. IIS Aragón. Zaragoza. Spain
Tel.: +34651739434
Correo electrónico: mbmedranoaragon@gmail.com
Acta Gastroenterol Latinoam 2019;49(1): 54-64
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE