Liliana Sampaio Costa Mendes,1 Barbara Castro Neves,2 Ana Loisa Silva de Menezes,3 Marcos Vasconcelos Carneiro,1 Jose Eduardo Trevizoli,1 Pauline Nallim Lobão,3 Rafael Rodrigues Oliveira,2 Wladimir Magalhães de Freitas4
1Hospital de Base do Distrito Federal.
2Universidade Católica de Brasília.
3Faculdades Integradas da União Educacional do Planalto Central.
4Biocárdios.
Brasília, DF, Brazil.
Acta Gastroenterol Latinoam 2020;50(3):264-270
Recibido: 26/03/2019 / Aprobado: 07/04/2020 / Publicado en www.actagastro.org el 28/09/2020
Summary
Introduction. Variable prevalence rates have been reported for Low bone mineral density (LBMD). Objectives. To determine the prevalence of LBMD in cirrhotic patients from a hospital outpatient clinic, to identify clinical and laboratory predictors of LBMD, and to determine the mean age at which LBMD was detected in this population. Methods. We conduced a cross-sectional study with 97 patients with liver cirrhosis for the presence of LBMD using bone densitometry of the lumbar spine and femoral neck. The prevalence of LBMD and mean age at detection of LBMD were evaluated. Correlation of LBMD with clinical-laboratory data was assessed, and uni and multivariate statistical analysis was performed. Results. The prevalence of LBMD was 50.5% in the 97 of the cases. LBMD was more frequent in participants over 50 years old (p = 0.001). There were no significant associations between the presence of LBMD and body mass index (p = 0.9), CHILD-PUGH score (p = 0.23), levels of 25-OH vitamin D (p = 0.5), parathyroid hormone (p = 0.5), calcium (p = 0.1), phosphorus (p = 0.3), and current or past smoking (p = 0.7). Age over 50 years remained a predictor of hepatic osteodystrophy even after adjusting for the other variables. Discussion. In conclusion LBMD was prevalent in the cirrhotic population studied. The age at LBMD diagnosis was between 15 and 20 years less than the osteoporosis screening age of the non-cirrhotic population. There were no clinical-laboratory factors that increased the suspicion of LBMD. Further studies are needed to determine whether these findings can be extrapolated to the cirrhotic population.
Key words. Cirrhosis, osteoporosis, osteopenia.
La baja densidad mineral ósea se manifiesta en pacientes cirróticos 15 a 20 años antes de la edad habitual de búsqueda
Resumen
Introducción. La prevalencia reportada de baja densidad mineral ósea (BDMO) es variable. Este estudio buscó determinarla en cirróticos de un centro ambulatorio identificar los predictores clínicos y laboratoriales de BDMO, y determinar la edad media en la cual se detectó BDMO en esa población. Realizamos un estudio de corte transversal, con 97 pacientes con cirrosis hepática. Objetivo. Determinar la prevalencia y la edad promedio de detección de BDMO en pacientes cirróticos, utilizando densitometría ósea de la columna lumbar y cuello femoral. Métodos. Se analizó la correlación de BDMO con datos clínico-laboratoriales, y se realizó análisis uni y multivariado. Resultados. La prevalencia de BDMO fue del 50,5% en los 97 casos. BDMO fue más frecuente en participantes mayores de 50 años (p = 0,001). No hubo asociación significativa entre la presencia de BDMO e índice de masa corporal (p = 0,9), puntuación de CHILD-PUGH (p = 0,23), niveles de vitamina D 25-OH (p = 0,5), hormona paratiroidea (p = 0,5), calcio (p = 0,1), fosforo (p = 0,3), y tabaquismo actual o previo (p = 0,7). Edad mayor que 50 años se mantuvo como predictor de BDMO incluso después de haberse ajustado por las demás variables. Discusión. La BDMO fue frecuente en la población cirrótica estudiada. La edad al diagnóstico de BDMO fue entre 15 y 20 años menos que la edad de detección de osteoporosis de la población no cirrótica. No hubo factores de laboratorio clínico que aumentaran la sospecha de BDMO. Se necesitan más estudios para determinar si estos hallazgos pueden extrapolarse a la población cirrótica.
Palabras claves. Cirrosis, osteoporosis, osteopenia.
Abbreviations
LC: Liver cirrhosis.
BMD: Bone mineral density.
LBMD: Low bone mineral density.
PTH: Parathyroid hormone.
BMI: Body mass index.
Introduction
Liver cirrhosis (LC) is a chronic condition in which the hepatic parenchyma is replaced with fibrosis and regenerative nodules. As a consequence of cirrhosis, there is an imbalance in bone remodeling followed by a decrease in osteogenesis and greater bone resorption.1, 2 The controversy term hepatic osteodystrophy is used to describe various changes in bone mineral density (BMD) in patients with chronic liver disease.3 Low bone mineral density (LBMD), can manifest itself more commonly as osteoporosis but osteopenia and osteomalacia may also occur.1, 2, 4 LBMD has a great impact on the morbidity and quality of life of the affected patients, increasing bone fragility and risk of fractures, especially in the spine and hip.4, 5
There is some evidence that LBMD is associated with age, LC severity and etiology.1-3 The prevalence of LBMD has been reported to be around 20% in patients with viral hepatitis, 55% in cirrhotic patients, and 13 to 60% in patients with cholestasis.2, 6 Even though less information is available regarding patients with non cholestatic LC, a previous study has reported a high incidence of LBMD expressed as low bone mineral density – 97% in patients with alcoholic cirrhosis and 93.7% in those with viral cirrhosis.6 High impact guidelines recommend the investigation of LBMD in all patients with cirrhosis, but this is not yet a reality, so the actual prevalence of LBMD remains unknown in this population.7 The aim of this study was to determine the prevalence of LBMD in a cirrhotic sample, determine the mean age at LBMD diagnosis in this population and associated factors with LBMD.
Methods
We performed a cross-sectional analysis of a convenience sample of patients with LC of different etiologies from a hepatology outpatient clinic for the presence of LBMD using bone densitometry from January to December 2018. The study was conducted at the Hospital de Base Institute of the Federal District, in Brasilia, Brazil.
Inclusion criteria were age ≥ 18 years and presence of LC of any etiology and severity. Exclusion criteria were hepatocarcinoma or any cancer, use of medications associated with bone loss, hospitalization in the last 30 days, and refusal to participate or to sign the consent form. Patient inclusion occurred after signature of an informed consent form. All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the institution’s ethics committee (CAAE ethics certificate: 3475.3314.0.0000.5553).
There were included 125 cirrhotic patients who attended the outpatient clinic for hepatology due to evaluation. We analyzed 97 who met the inclusion criteria and did not have exclusion criteria. Twenty-two patients were excluded from the analysis because they did not perform bone densitometry.
Data on the prevalence of LBMD and mean age at LBMD detection in this population were analyzed. We evaluated the correlation of LBMD with LC etiology and CHILD-PUGH score, sex, age, serum levels of 25-OH vitamin D, parathyroid hormone (PTH), calcium and phosphorus, current or past smoking, presence of ascites, and body mass index (BMI).
BMD analysis was performed with the Explorer QDR bone densitometer, (Hologic, Inc., Marlborough, MA) with evaluation of T score and Z score of the lumbar spine and femoral neck according to the guidelines of the Brazilian Society for Clinical Densitometry. Osteoporosis was defined in menopausal women and men over 50 years of age as a T score in BMD in the hip and/or spine ≥ 2.5 standard deviations from the normal adult value (T score ≤ -2.5). Osteopenia was defined in menopausal women and men over 50 years as a T score between -1 and -2.49. For men less than 50 years old and in non-menopausal women, the World Health Organization criterion of Z score ≤ -2 was used to define low BMD.8, 9 LBMD was diagnosed in the presence of osteoporosis, osteopenia, or low BMD in cirrhotic patients bellow 50 years or non-menopausal women when it´s not possible to rank into osteopenia or osteoporosis by T score but done through the Z score.
Statistical analysis
Continuous variables with normal and non-normal distribution were presented by the mean (standard deviation) and median (interquartile range) and categorical variables by percentage. Categorical variables were compared using the chi-square test and continuous variables using a t-test or Mann-Whitney test depending on the distribution. Study participants were categorized according to the LBMD. The univariate analysis was performed between LBMD and the following variables: LC etiology (viral and non-viral), CHILD-PUGH score, gender, age, serum levels of 25-OH vitamin D, parathyroid hormone (PTH), calcium, phosphorus, current or past smoking, presence of ascites, and body mass index (BMI).
p < 0.05 was considered statistically significant.
In a multivariate binary logistic regression model with LBMD as dependent variable, independent variables were incorporated according to clinical relevance and/or those that had an association with LBMD in the univariate models. The assumptions of linearity and absence of multi-collinearity were fulfilled. The linearity of the continuous variables with the logit of the dependent variable was evaluated by the Box-Tidwell procedure. In addition, all continuous independent variables were linearly related to the logit of the dependent variable. There was no residual greater than 2.5, and all cases were kept in the analysis. A multi-collinearity test was performed, and none of the independent variables showed a tolerance lower than 0.2 or VIF higher than 4.0, and all independent variables were added to the model.
Results
The baseline characteristics of the sample are shown in table 1. The mean age was 52.2 ± 13.5 years, and there was a predominance of males (56.7%). There was 33 menopausal women and 31 men over 50 in the studied population and 12 women under 50 and 21 men under 50 in the analyzed sample. Twenty-nine of the 33 menopausal women had LBMD while 4 of the 12 cirrhotic non menopausal women had LBMD. Two of the 21 men below 50 years old presented with LBMD while 16 of the 31 men over 50 presented with LBMD.
Table 1. Baseline characteristics of the sample.
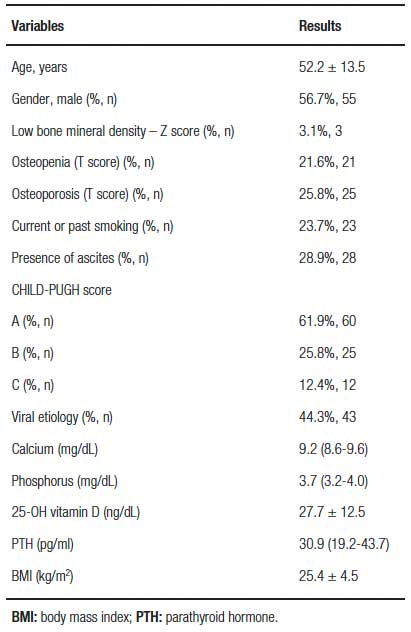
The viral etiology of cirrhosis predominated (44.3%), alcoholic and steatohepatitis non-alcoholic were in 23.7% (23 patients), autoimmune hepatitis in 13.4% and other etiologies in 18.6%. Most patients were classified as CHILD-PUGH A. Sixty patients were CHILD-PUGH A, 25 were CHILD-PUGH B and 12 were CHILD-PUGH C. All patients had laboratory tests for calcium, phosphorus, and PTH within normal limits. The mean 25-OH vitamin D level found in the population was 27.7 ± 12.5mg/dL. LBMD was detected in 49 of the 97 patients studied. In accordance with LBDM, only age tested as statistically significant in the univariate model. The variables gender, IMC, smoke, viral LC, ascites, CHILD-PUGH, Vitamin D, Calcium, Phosphorus and PTH were not statistically significant in the univariate model as show in table 2.
Table 2. Univariated analysis model.

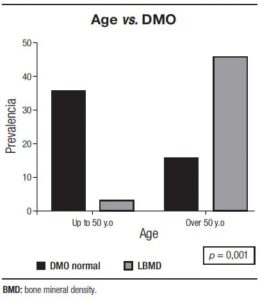
There were more cases of LBMD in patients over 50 years (p = 0.001) (Figure 1).
Figure 1. Low bone density in cirrhotic and age.
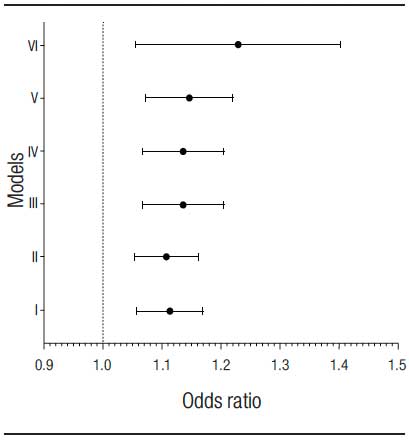
Multivariate analysis was performed using LBMD as a dependent variable and adjustments were made according to the models: I) adjusted for age, model II) adjusted for age and sex, model III) adjusted for age, sex and smoking, model IV) adjusted for age, sex, tobacco, CHILD-PUGH, ascites and body mass index. Model V was adjusted for age, sex, smoking, CHILD-PUGH, ascites and body mass index and viral etiology, while model VI was adjusted for age, sex, smoking, CHILD-PUGH, ascites and body mass index, etiology. viral, 25-OH vitamin D, calcium, phosphorus and parathyroid hormone. An age of more than 50 remained as a LBMD predictor even after adjusting for gender, smoking, ascites, CHILD-PUGH, BMI, etiology of LC, calcium, phosphorus, PTH and 25-OH vitamin D, according to the multivariate analysis models described in table 3, as shown in figure 2.
Table 3. Multivariate analysis models.
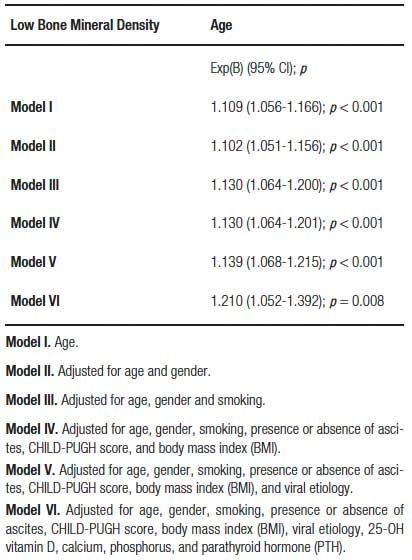
Figure 2. Absence of association of known risk factors in the literature with low bone density in the study population.
Discussion
Bone metabolism is altered in chronic liver disease, where there is a decrease in osteoblastic activity or an increase in osteoclastic activity, favoring greater bone loss.1 Alcohol consumption and iron overload reduce osteoblastic activity and may lead to hypogonadism, which in turn both reduces the half-life of osteoblasts and increases the half-life of osteoclasts.1, 10 Hyperbilirubinemia and vitamin D deficiency lower osteoblastic activity.1 LC is usually associated with decreased vitamin K activity, which leads to lower osteoclast apoptosis and decreased production of osteocalcin and osteonectin, which also increases osteoclastic activity.1 In LC of autoimmune origin, the use of corticosteroids, which increase interleukins 1 and 6, is usually indicated, causing greater osteoclastic activity. Corticosteroids also reduce the half-life and recruitment of osteoblasts, with a negative effect on bone mineralization.1, 10, 11
Prevalence data showed that LBMD occurred in 49 of the 97 (50.5%) cirrhotic patients evaluated. This prevalence is in agreement with data from the literature, which reports the occurrence of LBMD in 20 to 60% of patients with chronic liver disease.12 The great heterogeneity in case selection and divergent notions of LBMD in cross-sectional and longitudinal studies undermine the true prevalence estimate of LBMD in LC.4, 12
Smoking was not found to be a predictor of LBMD in the univariated analysis (p = 0.75) and it was also not statistically significant in the logistic regression models employed (III, IV, V and VI) in contrast to findings presented in the literature.13, 14 Perhaps the low number of smokers in this sample affected this evaluation. Labio and coworkers reported that ascites correlated with LBMD.15 In the present study, ascites was present in 28.9%, not being statistically significant in the univariate model (p = 0.94) and, due to its clinical relevance, it was included in the logistic regression but did not change the statistical relevance of age.
There was no association between BMI and LBMD. The data in the literature are also unclear regarding this association, not demonstrating a linear relationship between BMD and BMI, where both low BMI and obesity have been reported to be risk factors for bone mass decrease.14, 16, 17 The gender most correlated with LBMD is female, after menopause.18 In the sample, no statistical significance was shown for females in univariated analysis and when adjustment models were applied in multivariate analysis. Bone loss in LC might occur prior to menopause, since there are multiple factors related to the inhibition of bone formation by osteoblasts in this chronic disease.1, 18 Thus, LBMD could be manifested at ages younger than that recommended for screening of osteoporosis in the non-cirrhotic population, and the hormonal factor may have less impact on bone loss.
In this study, the variables serum levels of PTH, phosphorus, calcium, and 25-OH vitamin D were not associated with LBMD, neither in univariate and multivariate analyses nor when adjusted for the other risk factors in the multivariate analysis for the age of 50 years. This is probably due to the difference in the pathophysiology of LBMD and probable low prediction of osteoporosis and osteopenia through laboratory parameters. Vitamin D metabolism is normal in LBMD, although malabsorption of calcium and vitamin D may occur and lead to changes in skeletal bone.3 Hepatic hydroxylation is probably maintained, since in LBMD, supplementation is able to increase serum levels of vitamin D.10 In our series, all patients had vitamin D bellow 30 ng/mL, and there was no cut-off point capable of predicting the presence of LBMD, which has also been described in other studies.4, 6 The Institute of Medicine suggested that levels of vitamin D bellow 30 nmol/L (< 12 ng/mL) are considered for risk deficiency, at levels ranging from 30–50 nmol/L (12–20 ng/mL) people are potentially at risk for inadequacy but practically all people are sufficient at levels ≥ 50 nmol/L (≥ 20 ng/mL).19
The age over 50 was the only predictor of LBMD in our sample, there is no indication for routine screening for bone mass assessment in the general population in that age if there are no other risk factors, such as history of fracture, fragility syndrome, and use of medications that lead to loss of bone mass, among others, according to the Brazilian Society of Densitometry. This draws attention to the need to refer all cirrhotic patients for LBMD testing, regardless of age.9
Despite the recommendation to perform bone densitometry in every patient with LC, according to the American Gastroenterology Association,5, 20 the general guidelines of the Brazilian Society of Densitometry are to perform bone densitometry with the intention to screen women over 64 years, women aged 40 years or older in menopausal transition, and men aged 70 years or over. There is no mention of differential evaluation for cirrhotic patients.4, 6, 9, 21 Therefore, patients with LC may be at higher risk of fractures because they are not diagnosed early and do not receive adequate treatment for the disease, being susceptible to death or permanent physical disability.
In addition, about 50% of the patients develop bone changes 1 year after transplantation, as a result of immunosuppression and a long period of immobilization. However, most of these patients had altered bone metabolism prior to transplantation, thus developing an even greater risk of osteodystrophy.3 Therefore, it is important to identify these risk factors before transplantation to prevent osteoporosis in patients who were exposed to risk factors.22
In conclusion LBMD was prevalent in the cirrhotic population studied. The age at LBMD diagnosis was between 15 and 20 years less than the osteoporosis screening age of the non-cirrhotic population. There were no clinical-laboratory factors that increased the suspicion of LBMD. Further studies are needed to determine whether these findings can be extrapolated.
Acknowledgments. The authors thank Biocárdios, Brasília, DF, Brazil, for the support in the statistical analysis.
Funding source. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest. The authors declare that there is no conflict of interest.
Ethical disclosures. Protection of human and animal subjects: The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center in relation to the publication of patient data.
Right to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
References
- Goral V, Simsek M, Mete N. Hepatic osteodystrophy and liver cirrhosis. World J Gastroenterol 2010; 13: 1639-1643.
- Guarino M, Loperto I, Camera S, Cossiga V, Di Somma C, Colao A, Caporaso N, Morisco F. Osteoporosis across chronic liver disease. Osteoporos Int 2016; 27: 1967-1977.
- López-Larramona G, Lucendo AJ, González-Castillo S, Tenias JM. Hepatic osteodystrophy: An important matter for consideration in chronic liver disease. World J Hepatol 2011; 3: 300-307.
- Chinnaratha MA, Chaudhary S, Doogue M, McCormick RJ, Woodman RJ, Wigg AJ. Prevalence of hepatic osteodystrophy and vitamin D deficiency in cirrhosis. Intern Med J 2015; 45: 1230-1235.
- Leslie WD, Bernstein C, Leboff MS. AGA Technical review on osteoporosis in hepatic disorders. Gastroenterology 2003; 125: 941-966.
- Choudhary NS, Tomar M, Chawla YK, Bhadada SK, Khandelwal N, Dhiman RK, Duseja A, Bhansali A. Hepatic osteodystrophy is common in patients with non-cholestatic liver disease. Dige Dis Sci 2011; 56: 3323-3327.
- Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut 2002; 50: 1-9.
- Kanis JA. Assessment of fracture risk and its implication to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994; 4: 368-381.
- Brandão CM, Camargos BM, Zerbini CA, Plapler PG, Mendonça LM, Albergaria BH, Pinheiro MM, Prado Md, Eis SR. [2008 official positions of the Brazilian Society for Clinical Densitometry–SBDens]. Arq Bras Endocrinol Metab 2009; 53: 107-112.
- Gatta A, Verardo A, Di Pascoli M, Giannini S, Bolognesi M. Hepatic osteodystrophy. Clin Cases Min Bone Metab 2014; 11: 185-191.
- Bandgar T, Shivane V, Lila A, Shah N. Chronic liver disease and skeletal health (hepatic osteodystrophy). J Postgrad Med 2012; 58: 103-106.
- Goel V, Kar P. Hepatic osteodystrophy. Trop Gastroenterol 2010; 31: 82-86.
- Lupsa BC, Insogna K. Bone Health and Osteoporosis. Endocrinol Metab Clin North Am 2015; 44: 517-530.
- Christianson MS, Shen W. Osteoporosis prevention and management: non-pharmacologic and lifestyle options. Clin Obstet Gynecol 2013; 56: 703-710.
- Labio ED, Del Rosario DB, Strasser SI, McCaughan GW, Crawford BA. Effect of ascites on bone density measurement in cirrhosis. J Clin Densitom 2007; 10: 391-394.
- Skrzek A, Koziel S, Ignasiak Z. The optimal value of BMI for the lowest risk of osteoporosis in postmenopausal women aged 40-88 years. Homo 2014; 65: 232-239.
- Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Harrington LM, Breggia A, Rosen CJ, Miller KK. Determinants of bone mineral density in obese premenopausal women. Bone 2011; 48: 748-754.
- de Souza MP. Osteoporosis diagnosis and treatment. Rev Bras Ortop 2010; 45: 220-229.
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010.
- Gonçalves LL. Doença óssea na cirrose hepática. In: Farias AQ, de Souza AFM, editors. Doença óssea na cirrose hepática. São Paulo; Sociedade Brasileira de Hepatologia, 2010: 3-6. Available from: http://sbhepatologia.org.br/fasciculos/16.pdf. Accessed 2018 Apr 6.
- NIH consensus development panel on osteoporosis prevention, diagnosis and therapy. JAMA 2001; 285: 785-795.
- Crosbie OM, Freaney R, McKenna MJ, Curry MP, Hegarty JE. Predicting bone loss following orthotopic liver transplantation. Gut 1999; 44: 430-434.
Correspondencia: Liliana Sampaio Costa Mendes
SQS 312, bloco F, apto. 402, Asa Sul 70365-060. Brasília, DF, Brazil
Tel.: +55-61-984013142
Correo electrónico: mendesliliana2@gmail.com
Acta Gastroenterol Latinoam 2020;50(3):264-270
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE