Marina Jarschel Souza1 ID· Jessica Goedert Pereira1 ID· Ana Carolina de Souza Mangrich1 ID · Ana Paula Beltrame Farina Pasinato2 ID· Esther Buzaglo Dantas-Corrêa1 ID · Leonardo de Lucca Schiavon1 ID· Janaína Luz Narciso-Schiavon1 ID
1 Gastroenterology, Department of Internal Medicine – Federal University of Santa Catarina. Brasil.
2 Pathology Department – Hospital Universitário Polydoro Ernani de São Thiago – Universidade Federal de Santa Catarina, Florianópolis, SC, Brasil.
Acta Gastroenterol Latinoam 2023;53(1):39-48
Received: 13/09/2022 / Accepted: 22/02/2023 / Published online: 30/03/2023 / https://doi.org/10.52787/agl.v53i1.255
Summary
Introduction. Nonalcoholic fatty liver disease includes conditions such as nonalcoholic steatosis and nonalcoholic steatohepatitis, with varying degrees of fibrosis that can progress to cirrhosis and hepatocellular carcinoma. Although the gold standard for its diagnosis remains liver biopsy, many non-invasive methods have been proposed to aid in both the diagnosis of the disease and the evaluation of the presence of liver fibrosis, which is a strong and independent factor for liver related mortality. Objectives. The objectives of this study were: 1) to identify the clinical and laboratory features associated with the presence of advanced fibrosis in individuals with biopsy-confirmed nonalcoholic fatty liver disease; 2) to evaluate the performance of non-invasive markers in identifying patients with advanced fibrosis. Methods. A cross-sectional-analytic study that evaluated patients with nonalcoholic fatty liver disease treated in the outpatient clinic of a university hospital of reference in hepatology, between January 2013 and December 2016. Results. 81 patients aged 53.3 ± 9.8 years were included in this study; 39.5% were men and 70.1% were obese. When comparing patients with advanced fibrosis to those without advanced fibrosis, patients with advanced fibrosis had a lower proportion of males than females (17.6 vs. 45.4%, p = 0.038), a higher proportion of hypothyroidism (29.4 vs. 6.3%, p = 0.017) and a higher median AST (52 vs. 31 U/L, p = 0.005). In logistic regression analysis, only hypothyroidism was independently associated with advanced fibrosis (OR = 4.975; CI 95% 1.050 –
23.574; p = 0.043). Spearman correlation analysis showed that higher levels of fibrosis on liver biopsy, were associated with higher levels of TSH (r = 0.304; p = 0.036), AST (r = 0.277; p = 0.019), GGT (r = 0.284; p = 0.017) and LDL (r = 0.258; p = 0.037). Regarding the performance of the non-invasive markers, the area under the ROC curve of Fibrosis 4 score was 0.723 (p = 0.008), that of Nonalcoholic fatty liver disease fibrosis score was 0.713 (p = 0.022), that of gamma-glutamyl transferase platelet ratio was 0.697 (p = 0.019) and that of aspartate-to-alanine aminotransferase ratio was 0.689 (p = 0.031). Conclusions. Hypothyroidism is a factor independently associated with advanced fibrosis. In the outpatient setting, non-invasive markers may be useful in identifying patients with advanced fibrosis.
Keywords. Fatty liver, nonalcoholic fatty liver disease, liver fibrosis, liver cirrhosis, obesity, hypothyroidism.
Factores asociados a la fibrosis avanzada en pacientes con enfermedad hepática grasa no alcohólica
Resumen
Introducción. La enfermedad por hígado graso no alcohólico incluye condiciones tales como la esteatosis no alcohólica y la esteatohepatitis no alcohólica, con diversos grados de fibrosis que pueden progresar a cirrosis y carcinoma hepatocelular. Si bien el estándar de oro para su diagnóstico sigue siendo la biopsia hepática, se han propuesto muchos métodos no invasivos para ayudar tanto en el diagnóstico de la enfermedad como en la evaluación de la presencia de fibrosis hepática, factor fuerte e independiente de mortalidad relacionada con el hígado. Objetivos. Los objetivos de este estudio fueron: 1) identificar las características clínicas y de laboratorio asociadas a la presencia de fibrosis avanzada en individuos con enfermedad por hígado graso no alcohólico confirmada por biopsia, 2) evaluar el desempeño de marcadores no invasivos para identificar a los pacientes con fibrosis avanzada. Métodos. Estudio transversal, analítico, en el que se evaluaron pacientes con hígado graso no alcohólico atendidos en la consulta externa de un hospital universitario de referencia en hepatología, entre enero de 2013 y diciembre de 2016. Resultados. En el estudio se incluyeron 81 pacientes de 53,3 ± 9,8 años. El 39,5% eran hombres y el 70,1% eran obesos. Al comparar pacientes con fibrosis avanzada con aquellos sin fibrosis avanzada, los pacientes con fibrosis avanzada tenían una menor proporción de varones que de mujeres (17,6% vs. 45,4%, p = 0,038), una mayor proporción de hipotiroidismo (29,4% vs. 6,3%, p = 0,017) y unar mediana de AST más alta (52 vs. 31 U/L; p = 0,005). En el análisis de regresión logística, solo el hipotiroidismo se asoció de forma independiente con la fibrosis avanzada (OR = 4,975; IC 95% 1,050 – 23,574; p = 0,043). El análisis de correlación de Spearman mostró que los niveles más altos de fibrosis en la biopsia hepática se asociaron con niveles más altos de la Hormona estimulante de la tiroides (r = 0,304; p = 0,036), Aspartato aminotransferasa (r = 0,277; p = 0,019), Gamma-glutamil transferasa (r = 0,284; p = 0,017) y Lipoproteína de baja densidad (r = 0.258; p = 0.037). En cuanto al desempeño de los marcadores no invasivos, el área bajo la curva ROC del puntaje Fibrosis 4 fue 0,723 (p = 0,008), la del puntaje de fibrosis en la enfermedad por hígado graso no alcoholico fue 0,713 (p = 0,022), la de la relación de plaquetas/ gamma glutamil transferasa fue 0,697 (p = 0,019), y de la relación de aspartato/ alanina aminotransferasa fue de 0,689 (p = 0,031). Conclusiones. El hipotiroidismo es un factor que se asocia de forma independiente a la presencia de fibrosis avanzada. En la práctica ambulatoria, los marcadores no invasivos pueden ser de utilidad para identificar a los pacientes con fibrosis avanzada.
Palabras claves. Hígado graso, enfermedad hepática grasa no alcohólica, fibrosis hepática, cirrosis hepática, obesidad, hipotiroidismo.
Abbreviations
NAFLD: Nonalcoholic fatty liver disease.
NASH: Nonalcoholic steatohepatitis.
T2DM: Type 2 diabetes mellitus.
SAH: Systemic arterial hypertension.
AAR: Aspartate-to-alanine aminotransferase ratio.
APRI: Aspartate aminotransferase to platelet ratio index.
FIB-4: Fibrosis 4.
NFS: Nonalcoholic fatty liver disease fibrosis score.
HU/UFSC: Polydoro Ernani University Hospital of São Thiago, Federal University of Santa Catarina.
SPSS: Statistical Package for the Social Science.
BMI: Body mass index.
Hb: Hemoglobin.
AST: Aspartate aminotransferase.
ALT: Alanine aminotransferase.
ALP: Alkaline phosphatase.
GGT: Gamma-glutamyl transferase.
DB: Direct bilirubin.
PTA: Prothrombin time activity.
INR: International normalized ratio.
HbA1c: Glycosylated hemoglobin.
TSH: Thyroid stimulating hormone.
TG: Triglycerides.
TC: Total cholesterol.
HDL: High-density lipoprotein.
LDL: Low-density lipoprotein.
GPR: Gamma-glutamyl transferase platelet ratio.
AUROC: Area under the receiver operating characteristic curve.
NAS: NAFLD Activity score.
TSHR: Thyrotropin receptor.
T4: Thyroxine.
HBV: Hepatitis B virus.
HIV: Human immunodeficiency virus.
HCV: Hepatitis C virus.
Introduction
Nonalcoholic fatty liver disease (NAFLD) affects 17-46% of adults and is the most common disorder in Western countries.1 Nonalcoholic steatohepatitis (NASH) is present in up to 10% of individuals.2 NAFLD is currently the second most common indication for liver transplantation in the United States and is expected to become the first in the next 10 years.3 While the prevalence of other chronic liver diseases has remained stable or declined, the prevalence of NAFLD has doubled in the last 20 years.4
The clinical spectrum of NAFLD ranges from hepatic steatosis and NASH to cirrhosis and hepatocellular carcinoma.1, 5 Several conditions are clearly associated with the development of NAFLD, including obesity, type 2 diabetes mellitus (T2DM), systemic arterial hypertension (SAH), dyslipidemia and polycystic ovary syndrome. In addition, there are some conditions with an incipient association, such as hypothyroidism, obstructive sleep apnea and psoriasis, among others.5
Liver biopsy is essential to reliably differentiate between steatosis and steatohepatitis.1, 6 However, biopsy is an invasive procedure with risk of complications that depends on sample variability.6, 7 Thus, there is a great need for noninvasive methods that can aid in the diagnosis and prognosis of the disease. Many serum biomarkers attempt to assess the presence or absence of fibrosis, such as aspartate-to-alanine aminotransferase ratio (AAR), aspartate aminotransferase to platelet ratio index (APRI), fibrosis 4 (FIB-4), FibroTest, BARD score, and NAFLD fibrosis score (NFS). Some were developed primarily for other etiologies of cirrhosis, but they have been shown to be good predictors in the case of NAFLD.8-13
Therefore, the aim of this study was to identify clinical and laboratory features associated with the presence of advanced fibrosis in individuals with NAFLD and to evaluate the performance of non-invasive markers to identify patients with advanced fibrosis.
Methods
Cross-sectional analytical study conducted through review of medical records, evaluating individuals with NAFLD treated at the Gastroenterology and Hepatology Outpatient Clinic of the Polydoro Ernani University Hospital of São Thiago, Federal University of Santa Catarina (HU/UFSC), between January 2013 and December 2016, who underwent liver biopsy. Those with insufficient clinical and laboratory data in the medical records or other diagnoses as a cause of liver disease were excluded.
The clinical, laboratory and histological characteristics of the subjects included in the study were analyzed. All data were collected from medical records and anatomopathological reports, and transferred to the Statistical Package for the Social Science (SPSS), version 17 (Chicago; Illinois, USA).
The following clinical characteristics were evaluated: sex; age; body mass index (BMI); obesity; history of diabetes mellitus; dyslipidemia; alcohol abuse, defined as a report of alcohol intake greater than 20 g per day for women and 30 g per day for men;1, 6 SAH; hypothyroidism, defined by clinical history and considered only in individuals undergoing treatment.
Other variables evaluated were: skin color; liver failure; hemoglobin (Hb), platelets; aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), direct bilirubin (DB), serum albumin, prothrombin time activity (PTA), international normalized ratio (INR), creatinine, ferritin, glycemia, glycosylated hemoglobin (HbA1c), thyroid stimulating hormone (TSH), triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL).
The formulas used to calculate the AAR, GGT platelet ratio (GPR) and FIB-4 were: AAR=AST (IU/L) /
ALT (IU/L); GPR = (GGT (IU/L) / maximum GGT reference value (IU/L) / platelets (109/L) × 100; FIB-4 = age (years) × AST (IU/L) / (platelets (109/L) × (ALT (IU/L) / 2). The NFS was calculated using the online calculator http://gihep.com/calculators/hepatology/nafld-fibrosis-score/ and the thresholds for the NFS used were as follows: stage 1, ≤ -1.455 (predictor of the absence of significant fibrosis, fibrosis F0-F2); stage 2, > -1.455 to ≤ 0.675 (indeterminate score); stage 3, > 0.675 (predictor of the presence of significant fibrosis, fibrosis F3-F4).12 The predictive accuracies of the noninvasive models (FIB-4, NFS and GPR) were tested by measuring the areas under the receiver operating characteristic curves (AUROC).
Histological analysis
Only liver tissue containing at least ten portal spaces was selected for evaluation. The degree of fibrosis was scored from 0 to 4; 0 for absence, 1 for perisinusoidal or periportal, 2 for perisinusoidal and portal/periportal fibrosis, 3 for bridging fibrosis and 4 for cirrhosis.14 Advanced fibrosis was defined as fibrosis 3 or 4 (F3-F4). Based on the original histopathological criteria (necroinflammatory grade) of Brunt et al., each case was classified as mild (grade 1), moderate (grade 2), or severe (grade 3) NASH.15 Subsequently, for clinical evaluation and follow-up, disease severity was assessed according to the NAFLD activity score (NAS), which is the sum of the semiquantitative assessment of steatosis, ballooning and lobular inflammation, and can range from 0 to 8. Cases with NAS from 0 to 2 were widely considered non-diagnostic for steatohepatitis. On the other hand, most cases with scores ≥ 5 were diagnosed as steatohepatitis.14,16
Statistical analysis
Continuous variables were described using measures of central tendency and dispersion, while categorical variables were described using absolute numbers and proportions. Continuous variables were compared using Student’s t-test or Mann-Whitney test. When appropriate, categorical variables were compared using the chi-squared test or Fisher’s exact test. Bivariate analysis was performed to identify the characteristics associated with advanced fibrosis. Spearman’s correlation analysis was performed to identify continuous variables that correlated with the degree of liver fibrosis detected by biopsy. P values less than 0.05 were considered statistically significant. All tests used were two-tailed and performed by SPSS; version 17.0.
Results
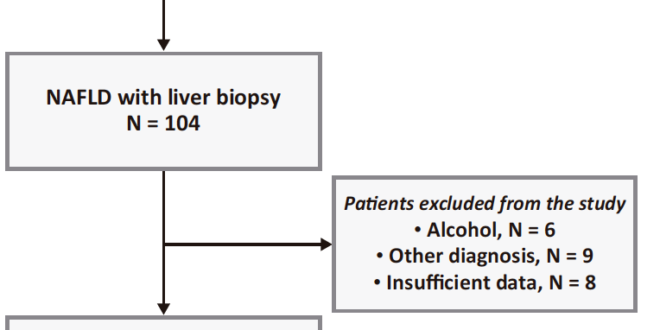
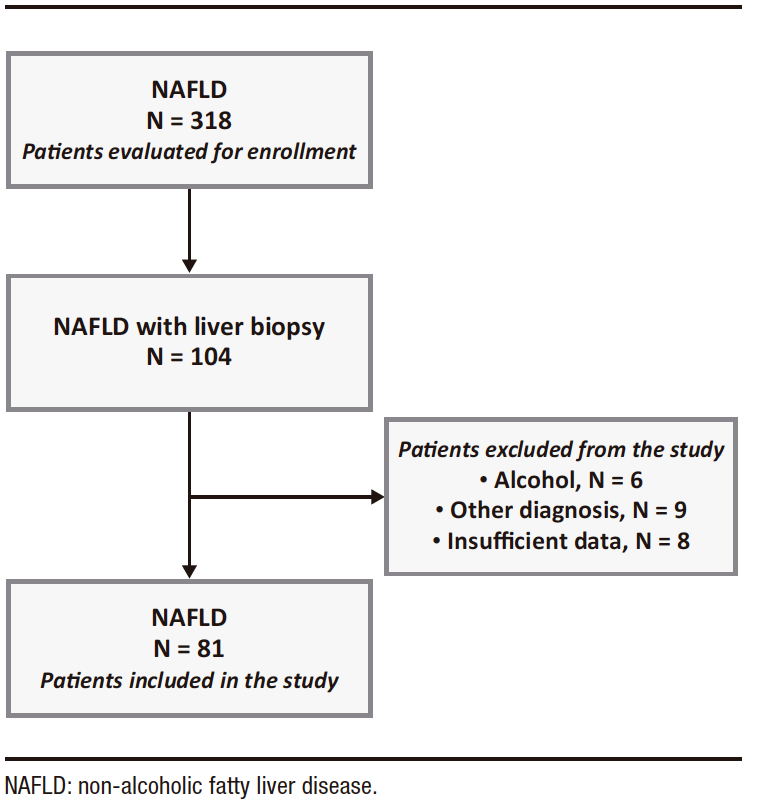
A total of 318 patients with ultrasound-detected steatosis treated during the study period were evaluated for inclusion. Of these, 104 underwent liver biopsy. The study excluded 6 patients with alcohol consumption greater than 30 g/day for men and 20 g/day for women, 9 with other diagnoses as the cause of liver disease, and 8 with insufficient data. (Figure 1)
Figure 1. Flowchart of included patients
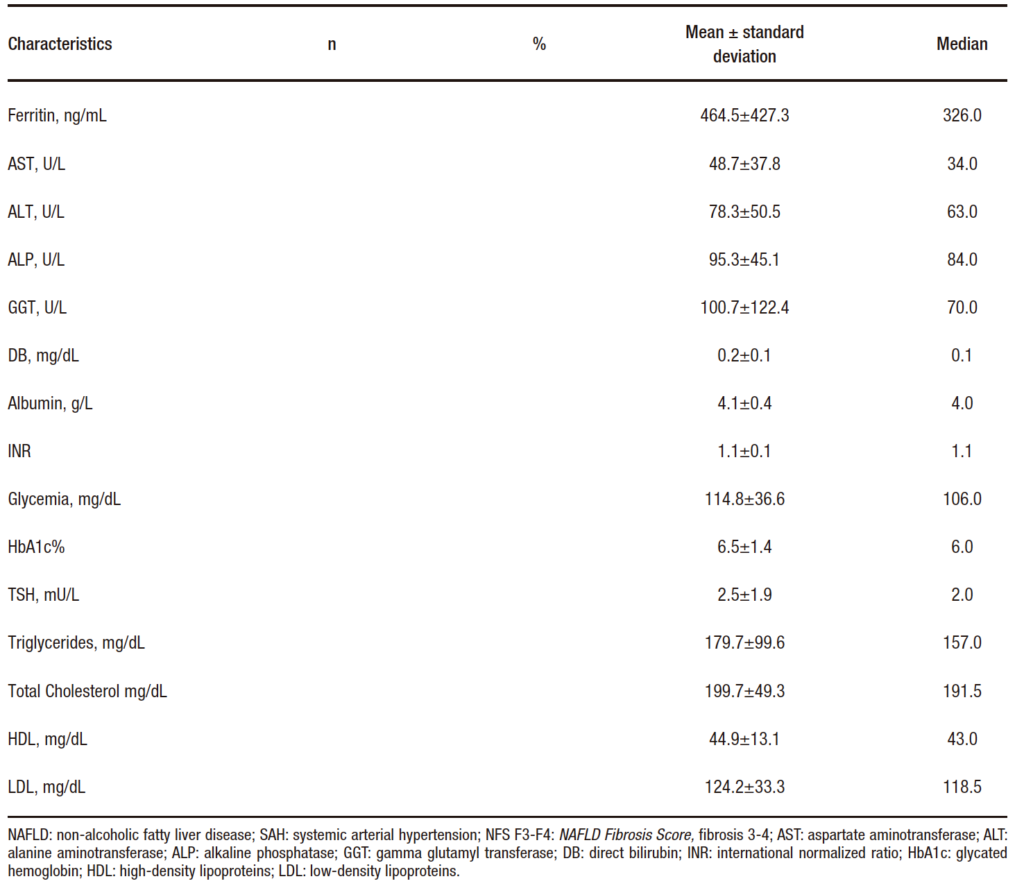
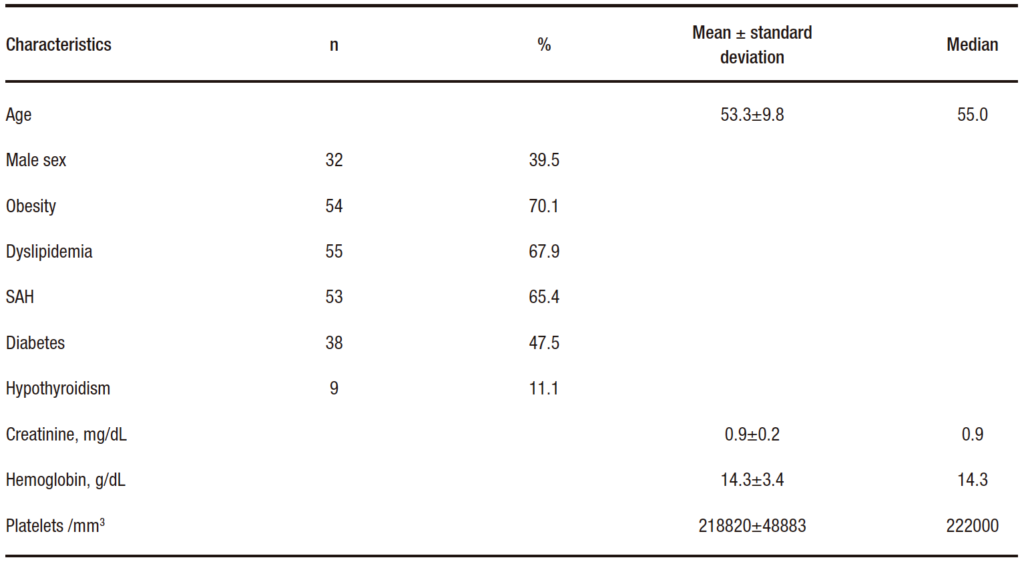
The study included 81 patients with a mean age of 53.3 ± 9.8 years. Of these, 39.5% were men; 70.1% were obese; 67.9% had dyslipidemia; 65.4% had hypertension; almost half of the participants (47.5%) had T2DM; and 11.1% of the participants had hypothyroidism. The clinical and laboratory characteristics of the patients are described in Table 1.
Table 1. Clinical and laboratory characteristics of 81 patients with NAFLD
When evaluating the anatomopathological reports of liver biopsies, 63 (77.8%) patients were found to have moderate or severe steatosis. Stage ≥ 2 steatohepatitis was detected in 71 patients (87.7%), 11 patients (13.6%) had NAS ≤ 2 and 41 patients (50.6%) had NAS ≥ 5. Ballooning was identified in 53 (65.4%) patients, inflammation in 66 (81.5%), siderosis in 24 (29.6%) and
Mallory’s bodies in 7 (8.6%). When evaluating the degree of fibrosis, 12 (14.8%) patients had grade 0, 30 (37.0%) grade 1, 12 (14.8%) grade 2, 14 (17.2%) grade 3 and 3 (3.7%) grade 4. Seventeen patients (21.0%) had advanced fibrosis.
When comparing individuals with advanced fibrosis to those without advanced fibrosis (Table 2), patients with advanced fibrosis had a lower proportion of males (17.6 vs. 45.4%; p = 0.038); a higher proportion of hypothyroidism (29.4 vs. 6.3 mU/L; p = 0.017); higher median AST (52 vs. 31 U/L; p = 0.005); higher mean AST/ALT ratio (0.76 ± 0.2 vs. 0.61 ± 0.3; p = 0.031) and higher median FIB-4 (1.6 vs. 1.1; p = 0.008), GPR (0.5 vs. 0.3; p = 0.019) and NFS scores of- 0.02 vs. – 1.26 (p = 0.022). There were no differences in age, BMI, T2DM, SAH, dyslipidemia, obesity, creatinine, hemoglobin, ferritin, TSH, ALT, ALP, GGT, DB, albumin, INR, glycemia, HbA1c, triglycerides, total cholesterol, HDL and LDL. The multivariate analysis included the variables that showed p < 0.050 in the univariate analysis and T2DM, which is classically associated with advanced fibrosis in NAFLD. As this was an exploratory analysis, the non-invasive models previously shown to be associated with fibrosis were not included.
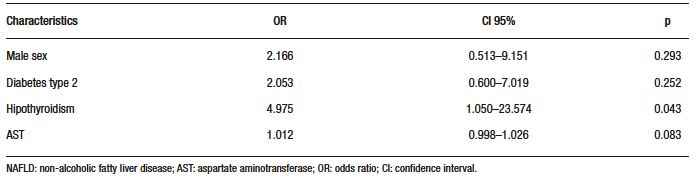
The results of the regression analysis are in Table 3. Only hypothyroidism was independently associated with advanced fibrosis (OR: 4.975; CI 95% 1.050-23.574;
p = 0.043).
Table 2. Comparative analysis of individuals with NAFLD according to the presence of advanced fibrosis on liver biopsy
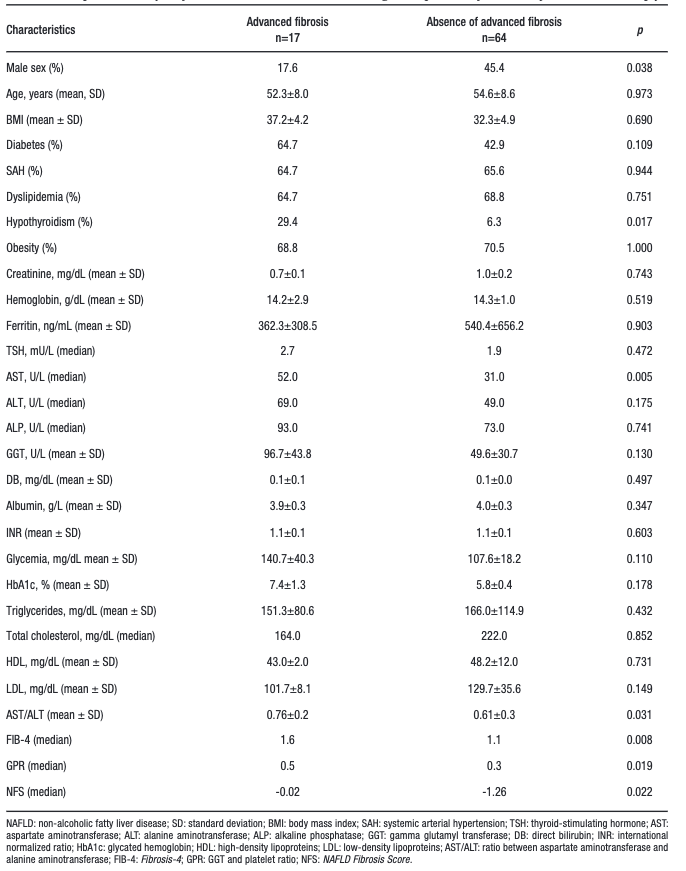
Table 3. Multivariate analysis of factors associated with the presence of advanced fibrosis on liver biopsy in 81 individuals with NAFLD
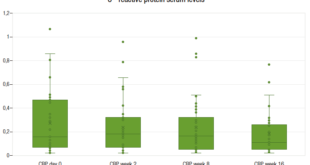
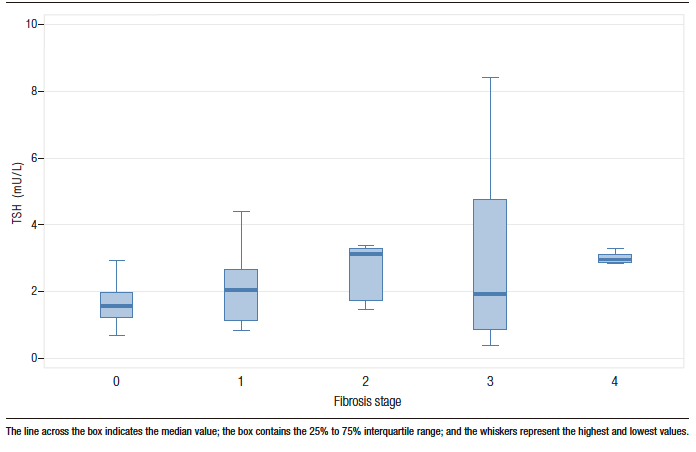
Spearman correlation analysis showed that the higher the degree of fibrosis at liver biopsy, the higher the TSH (r = 0.304; p = 0.036), AST (r = 0.277; p = 0.019), GGT (r = 0.284; p = 0.017), and LDL levels (r = 0.258; p = 0.037), and the higher the AAR (r = 0.309; p = 0.009), GPR (r = 0.319; p = 0.009) and FIB-4 (r = 0.320; p = 0.008) results of the individuals. Figure 2 shows TSH levels according to the stage of liver fibrosis. There was no correlation between the degree of liver fibrosis and the values of age, BMI, creatinine, hemoglobin, platelets, ferritin, ALT, ALP, BD, albumin, INR, glycemia, HbA1c, TG, TC, and HDL. There was also no correlation between fibrosis and NFS (r = – 0.092; p = 0.0502).
Figure 2. Box plot of TSH levels by stage of liver fibrosis
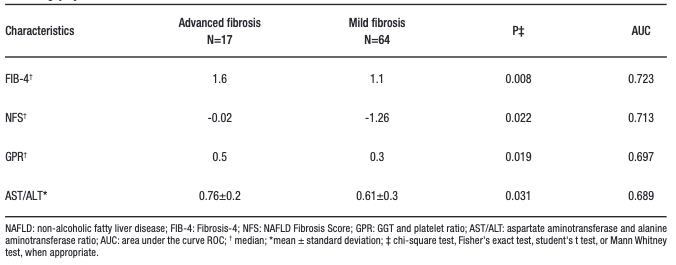
Regarding the performance of the non-invasive markers (Table 4), the AUROC of FIB-4 was 0.723
(p = 0.008), NFS was 0.713 (p = 0.022), GPR was 0.697 (p = 0.019) and AAR was 0.689 (p = 0.017).
Table 4. Comparative analysis and performance of non-invasive markers according to the presence of advanced fibrosis on liver biopsy of 81 individuals with NAFLD
Discussion
Our study showed a significant relationship between advanced fibrosis and the presence of hypothyroidism, and a correlation between higher TSH levels and higher fibrosis degree, a trend in the current literature.17-19 Conversion of thyroxine (T4) to triiodothyronine also occurs in the liver, which synthesizes thyroid hormone-binding lipophilic plasma proteins. Hepatocyte integrity is essential for the maintenance of thyroid function.19
Hypothyroidism can induce steatogenesis because thyroid hormones increase the expression of LDL receptors in hepatocytes and increase the activity of fat-reducing enzymes, thereby decreasing serum LDL levels.19 A recent meta-analysis17 of 26 studies showed that TSH may be an important risk factor for the development and progression of NAFLD, independent of thyroid hormones.
Even in subjects with normal TSH levels, NAFLD patients had significantly higher TSH levels than healthy controls, and TSH levels increased with NAFLD progression.17 Kim et al.18 showed that the risk of NASH and advanced fibrosis increased significantly with TSH levels. Subclinical hypothyroidism was significantly associated with the risk of NASH, even after adjustment for obesity, metabolic risk factors and insulin resistance, suggesting that subclinical hypothyroidism is an independent factor for NASH and advanced fibrosis.18 One hypotheses for this occurrence is the positive association between TSH concentrations and TG, even within the normal range. Another hypothesis relates to the fact that liver cells express the thyrotropin receptor (TSHR), and when TSH binds to this receptor in hepatocytes, it triggers a pathway associated with lipogenesis.17 TSRH may also be related to the fibrosing effect of TSH, TSRH α1 is predominant in hepatic stellate cells, suggesting that it may modulate fibrinogenesis. Whereas in liver cells, TSRHs α1 and β1 are suppressed in chronic liver injury.17 In addition, thyroid dysfunction may also mediate hepatic histological damage through oxidative stress and mitochondrial dysfunction.18
The present study shows a significant association between AST and AAR levels and advanced fibrosis.
In NAFLD, ALT levels are usually higher than AST levels, but with disease progression the relationship is reversed. Thus, an AAR > 1 indicates advanced fibrosing disease, which may be related to the reduction in sinusoidal AST clearance in relation to ALT.20 Many scores for assessing fibrosis already use AAR as a component: HSI, NLFS, BARD, and NFS.7 This ratio has a good negative predictive value and could be used to rule out the presence of advanced fibrosis.21 In adittion, it can be calculated by widely available laboratory tests commonly used in the follow-up of these patients.20-22 However, studies suggest that ALT may decrease not only in fibrosis but also with age; therefore, AAR should be used with caution in older adults.23
It has been suggested that GPR can evaluate liver fibrosis in patients with chronic hepatitis B and NAFLD.26, 27
In 2016, Lemoine et al.13 proposed GPR as a marker of fibrosis stage in patients with chronic hepatitis B. Li et al.26 evaluated GPR as a predictive marker of fibrosis compared to liver biopsy in patients with HBV and NAFLD (HBV-NAFLD). In this study, GGT levels were higher in patients with HBV-NAFLD than in patients with HBV alone. Our study showed higher GPR results in patients with advanced fibrosis and also a correlation between fibrosis levels and GPR in patients who had only NAFLD and did not have chronic hepatitis B. We found no other studies evaluating this score in patients with NAFLD alone. Further studies are needed in this area, as this is not a validated score for this purpose.
The FIB-4 score was primarily developed in 2006 to predict fibrosis in patients with HIV/HCV coinfection, using simple and available variables: age, AST, and platelets.9 Since then, it has been used to predict fibrosis in other diseases such as NAFLD.1, 5, 28, 29
In 2007, Angulo et al. created the NFS, developed specifically for this group of patients and also used to identify fibrosis.12 To calculate the NFS, easily accessible variables are also used: the presence of altered fasting glycemia or T2DM, age, AST, ALT, platelets, BMI, and albumin.12 In our study, we observed higher results for both scores in patients with advanced fibrosis and a correlation between increased FIB-4 values and increased fibrosis. Both NFS and FIB-4 have been validated in NAFLD populations with consistent results, and both predict all-cause mortality, cardiovascular mortality, and liver-related mortality.1 Moreover, they perform better in differentiating advanced fibrosis (≥ F3) and have higher negative predictive values for excluding advanced fibrosis than positive predictive values.1 NFS < -1.455 had 90% sensitivity and 60% specificity for excluding advanced fibrosis, while NFS > 0.676 had 67% sensitivity and 97% specificity for identifying advanced fibrosis.5 Compared with scores such as BARD, APRI, and AAR, NFS and FIB-4 perform better in predicting advanced fibrosis in patients with biopsy-confirmed NAFLD.5
Our study included 81 patients whose liver biopsies were analyzed by a single pathologist specializing in liver pathology. However, we must emphasize that this is not a prospective multicenter study. Nevertheless, data collection was standardized and the study took place in a reference center for liver diseases. Another limitation is that since the patients included in this study generally underwent a liver biopsy to evaluate aggressive or advanced disease, our results reflect a sicker population than general practice.
Conclusion
This study suggests that hypothyroidism may be an independent factor associated with the presence of advanced fibrosis in patients with NAFLD. If further studies confirm our findings, active screening for hypothyroidism in patients with NAFLD may be an important recommendation in clinical practice. In addition, our data indicate that non-invasive markers can be very useful in identifying patients with advanced fibrosis.
Consent for publication. Written informed consent was obtained from the patient or patient’s father, guardian or family member for publication of the data and/or clinical images for the benefit of science. A copy of the consent form is available to the editors of this journal.
Intellectual Property. The authors declare that the data, figures and tables presented in the manuscript are original and were carried out at their belonging institutions.
Funding. The authors declare that there were no external sources of funding.
Conflict of interest. The authors declare that they have no conflicts of interest in relation to this article.
Copyright
© 2023 Acta Gastroenterológica latinoamericana. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license, which allows non-commercial use, distribution, and reproduction, provided the original author and source are acknowledged.
Cite this article as: Jarschel Souza M, Goedert Pereira J, de Souza Mangrich A C et al. Factors Associated with Advanced Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Acta Gastroenterol Latinoam. 2023;53(1):39-48. https://doi.org/10.52787/agl.v53i1.255
References
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-402.
- Parker R, Hodson J, Rowe IAC. Systematic review: Current evidence in non-alcoholic fatty liver disease lacks relevance to patients with advanced fibrosis. J Gastroenterol Hepatol. 2017;32(5):950-6.
- Byrne CD, Targher G. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease: is universal screening appropriate? Diabetologia. 2016;59(6):1141-4.
- LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh K-L, et al. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48(6):467-73.
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-57.
- Shah RA, Kowdley KV. Serum ferritin as a biomarker for NAFLD: ready for prime time? 2019.
- Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156(5):1264-81.e4.
- Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple non-invasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518-26.
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple non-invasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317-25.
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357(9262):1069-75.
- Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118(6):1117-23.
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a non-invasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846-54.
- Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65(8):1369-76.
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313-21.
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467-74.
- Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810-20.
- Guo Z, Li M, Han B, Qi X. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig Liver Dis. 2018;50(11):1153-62.
- Kim D, Kim W, Joo SK, Bae JM, Kim JH, Ahmed A. Subclinical Hypothyroidism and Low-Normal Thyroid Function Are Associated With Nonalcoholic Steatohepatitis and Fibrosis. Clin Gastroenterol Hepatol. 2018;16(1):123-31.e1.
- Silva NdOe, Ronsoni MF, Colombo BdS, Corrêa CG, Hatanaka SA, Canalli MHBdS, et al. Clinical and laboratory characteristics of patients with thyroid diseases with and without alanine aminotransferase levels above the upper tertile Cross-sectional analytical study. Archives of Endocrinology and Metabolism. 2016;60:101-7.
- Guha IN, Parkes J, Roderick PR, Harris S, Rosenberg WM. Non-invasive markers associated with liver fibrosis in non-alcoholic fatty liver disease. Gut. 2006;55(11):1650-60.
- Alkhouri N, McCullough AJ. Non-invasive Diagnosis of NASH and Liver Fibrosis Within the Spectrum of NAFLD. Gastroenterol Hepatol (N Y). 2012;8(10):661-8.
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265-9.
- Goh GB-B, Pagadala MR, Dasarathy J, Unalp-Arida A, Pai RK, Yerian L, et al. Age impacts ability of aspartate-alanine aminotransferase ratio to predict advanced fibrosis in nonalcoholic Fatty liver disease. Dig Dis Sci. 2015;60(6):1825-31.
- Petta S, Macaluso FS, Barcellona MR, Camma C, Cabibi D, Di Marco V, et al. Serum gamma-glutamyl transferase levels, insulin resistance and liver fibrosis in patients with chronic liver diseases. PLoS One. 2012;7(12):e51165.
- Shukla A, Kapileswar S, Gogtay N, Joshi A, Dhore P, Shah C, et al. Simple biochemical parameters and a novel score correlate with absence of fibrosis in patients with nonalcoholic fatty liver disease. Indian J Gastroenterol. 2015;34(4):281-5.
- Li Q, Lu C, Li W, Huang Y, Chen L. The gamma-glutamyl transpeptidase to platelet ratio for non-invasive assessment of liver fibrosis in patients with chronic hepatitis B and non-alcoholic fatty liver disease. Oncotarget. 2017;8(17):28641-9.
- Schiavon LL, Narciso-Schiavon JL, Ferraz MLG, Silva AEB, Carvalho-Filho RJ. The gamma-glutamyl transpeptidase to platelet ratio (GPR) in HBV patients: just adding up? (1468-3288 (Electronic).
- Gómez de la Cuesta S, Aller de la Fuente R, Tafur Sánchez C, Izaola O, García Sánchez C, Mora N, et al. Factores analíticos, antropométricos y dietéticos asociados al desarrollo de fibrosis en pacientes con enfermedad por hígado graso no alcohólico. Rev esp enferm dig. 2018;110(5):292-8.
- Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology. 2017;66(5):1486-501.
Correspondence: Janaína Luz Narciso-Schiavon
Email:: janaina.narciso@uol.com.br
Acta Gastroenterol Latinoam 2023;53(1):39-48
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE