Nicolás Airaldi ID· Manuel García ID· Agustín R Gigena ID· Fernando Martínez Lascano ID Carlos M Esquivel ID
Sanatorio Allende de Córdoba.
Provincia de Córdoba, Argentina.
Acta Gastroenterol Latinoam 2023;53(4):361-368
Received: 31/10/2023 / Accepted: 21/12/2023 / Published online: 29/12/2023 /
https://doi.org/10.52787/agl.v53i4.360
Summary
Introduction. Obesity is a risk factor for gastrointestinal diseases such as gastroesophageal reflux disease, erosive esophagitis, and hiatal hernia, among others. The presence of some of these diseases during the preoperative bariatric study may condition the surgical technique to be performed. Materials and methods. We conducted a retrospective, descriptive, cross-sectional study from a prospectively collected database of patients with obesity treated with bariatric surgery between January 2016 and June 2022 who systematically underwent preoperative upper gastrointestinal endoscopy. Results. We evaluated 704 patients who were candidates for bariatric surgery. The mean age of the patients was 40 years, predominantly female (71.6%). The most frequent endoscopic findings were gastropathies, which appeared in 98.9% (n: 696) of the patients, followed by esophageal disorders in 11.6%
(n: 82). The prevalence of erosive esophagitis was 9.8% (n: 69). In addition, 19.7% of the endoscopic findings required preoperative medical treatment and in 4% of the patients the surgical decision was determined by the endoscopic finding. Conclusion. Given the high relationship between obesity and diseases of the upper digestive tract, and the interference that these diseases can have in the postoperative evolution of a bariatric patient, we consider that upper gastrointestinal endoscopy should be routinely included in the preparation of the patient who is a candidate for bariatric surgery.
Keywords. Bariatric surgery, bariatric endoscopy.
Hallazgos endoscópicos en pacientes candidatos a cirugía bariátrica
Resumen
Introducción. La obesidad es un factor de riesgo para enfermedades gastrointestinales como la enfermedad por reflujo gastroesofágico, la esofagitis erosiva y la hernia de hiato, entre otras. La presencia de algunas de estas enfermedades durante el estudio bariátrico preoperatorio puede condicionar la técnica quirúrgica a realizar. Sin embargo, la endoscopía prebariátrica sigue siendo objeto de debate en todo el mundo. Materiales y métodos. Se realizó un estudio retrospectivo, descriptivo y transversal a partir de una base de datos recopilada prospectivamente de pacientes con obesidad tratados con cirugía bariátrica entre enero de 2016 y junio de 2022 a los que se les realizó sistemáticamente una endoscopia gastrointestinal superior preoperatoria. Resultados. Se evaluaron 704 pacientes candidatos a cirugía bariátrica. La edad media de los pacientes fue de 40 años, predominando el sexo femenino (71,6%). Los hallazgos endoscópicos más frecuentes fueron las gastropatías, que aparecieron en el 98,9% (n: 696) de los pacientes, seguidas de las afecciones esofágicas en el 11,6% (n: 82). La prevalencia de esofagitis erosiva fue del 9,8% (n: 69). Además, se observó que el 19,7% de los hallazgos endoscópicos precisaban un tratamiento médico preoperatorio y en el 4% de los pacientes la decisión quirúrgica venía determinada por el hallazgo endoscópico. Conclusión. Dada la alta relación entre obesidad y enfermedades del tracto digestivo superior, y la interferencia que estas enfermedades pueden tener en la evolución postoperatoria de un paciente bariátrico, consideramos que la endoscopía digestiva alta debe ser incluida rutinariamente en la preparación del paciente candidato a cirugía bariátrica.
Palabras claves. Cirugía bariátrica, endoscopia bariátrica.
Abbreviations
WHO: World Health Organization.
RYGB: Roux-en-Y Gastric Bypass.
BE: Barret’s esophagus.
PPI: Proton pump inhibitors.
BMI: Body mass index.
Introduction
Obesity is a chronic disease, characterized by an increase in body fat. It is considered a worldwide epidemic by the World Health Organization (WHO), being the second leading cause of preventable death after smoking.1
In its multidisciplinary approach, bariatric surgery has shown greater efficacy regarding weight loss and control of comorbidities, and maintenance of these two variables in the long term, compared to multiple non-surgical treatments such as diet therapy, physical activity and pharmacotherapy.2
Obesity is a risk factor for gastrointestinal diseases such as gastroesophageal reflux disease, erosive esophagitis, and hiatal hernia, among others.3 The presence of some of these diseases, as well as the incidental finding of premalignant/malignant or ulcerative tumor lesions, may condition the surgical technique to be performed (gastric sleeve or gastric bypass).4 For this reason, mulmultiple studies on bariatric surgery recommend that all patients-candidates for obesity surgery should undergo a preoperative esophagogastroduodenal videoendoscopy.4-5 However, it is not an established standard of care in many bariatric centers.6
Hypothesis
Esophagogastroduodenal endoscopy allows us to define both the preoperative preparation and the surgical technique to be performed in patients who are candidates for bariatric surgery.
Objective
To analyze the presence of esophagogastroduodenal pathologies by preoperative esophagogastroduodenal videoendoscopy in patients-candidates for bariatric surgery.
Materials and Methods
A retrospective, descriptive, cross-sectional study from a prospectively collected database was performed. All 704 patients undergoing bariatric surgery between January 2016 and June 2022 who systematically underwent preoperative esophagogastroduodenal endoscopy were included.
Inclusion criteria were patients older than 18 years with preoperative endoscopic report in their medical record.
Exclusion criteria were patients with previous bariatric surgery and patients whose esophagogastroduodenal endoscopy was not performed in our institution.
The endoscopies were performed by the Gastroenterology and Digestive Endoscopy Department of Sanatorio Allende, following the department’s protocol, which consists of pre-surgical studies, cardiovascular risk assessment and 8-hour fasting prior to the procedure. The endoscopies were performed with the patient in left lateral decubitus position under Propofol sedation. The studies were performed with the Evis Exera III Olympus 190 videogastroscope. Los Angeles Classification was used to describe esophagitis. Biopsy samples were taken in all patients to detect the presence of Helicobacter pylori (2 samples from the antrum, 1 from the angular incisura and 1 from the body of the stomach) and to rule out villous atrophy (2 samples from the second duodenal portion).
All surgeries were performed by physicians of the staff Department of Bariatric and Metabolic Surgery of Sanatorio Allende, with the same surgical technique in the procedures performed (gastric bypass, resective gastric bypass, and gastric sleeve).
Variables evaluated included: abnormal endoscopic findings such as erosive esophagitis, congestive or erosive gastropathy, duodenitis (defined as erythema, erosions and/or sub-epithelial haemorrhage), ulcers, tumors and the presence of Helicobacter pylori; changes in the planned bariatric surgical procedure after endoscopy; and/or surgical technique determined by the endoscopic finding.
The presence of grade B, C or D esophagitis and/or hiatal hernia larger than 4 cm were considered an indication for Roux-en-Y gastric bypass (RYGB).
A descriptive statistical analysis was performed, wich is expressed in terms of frequency and percentages. The Rmedic program7 was used.
The study project was approved by the Institutional Health Research Ethics Committee. According to the WHO this project represents a low-risk study. It is a retrospective observational study whereby the informed consent was no needed.
Results
A total of 704 patients who were candidates for bariatric surgery were evaluated by esophagogastroduodenoscopies. The mean age of the patients was 40 years, predominantly female (71.6%). The most performed surgery was RYGB (96.3%) followed by sleeve gastrectomy (2.3%).
All the patients evaluated presented some endoscopic finding. The most frequent findings were gastropathies, which appeared in 696 patients (98.9%), followed by esophageal affections in 82 patients (11.6%).
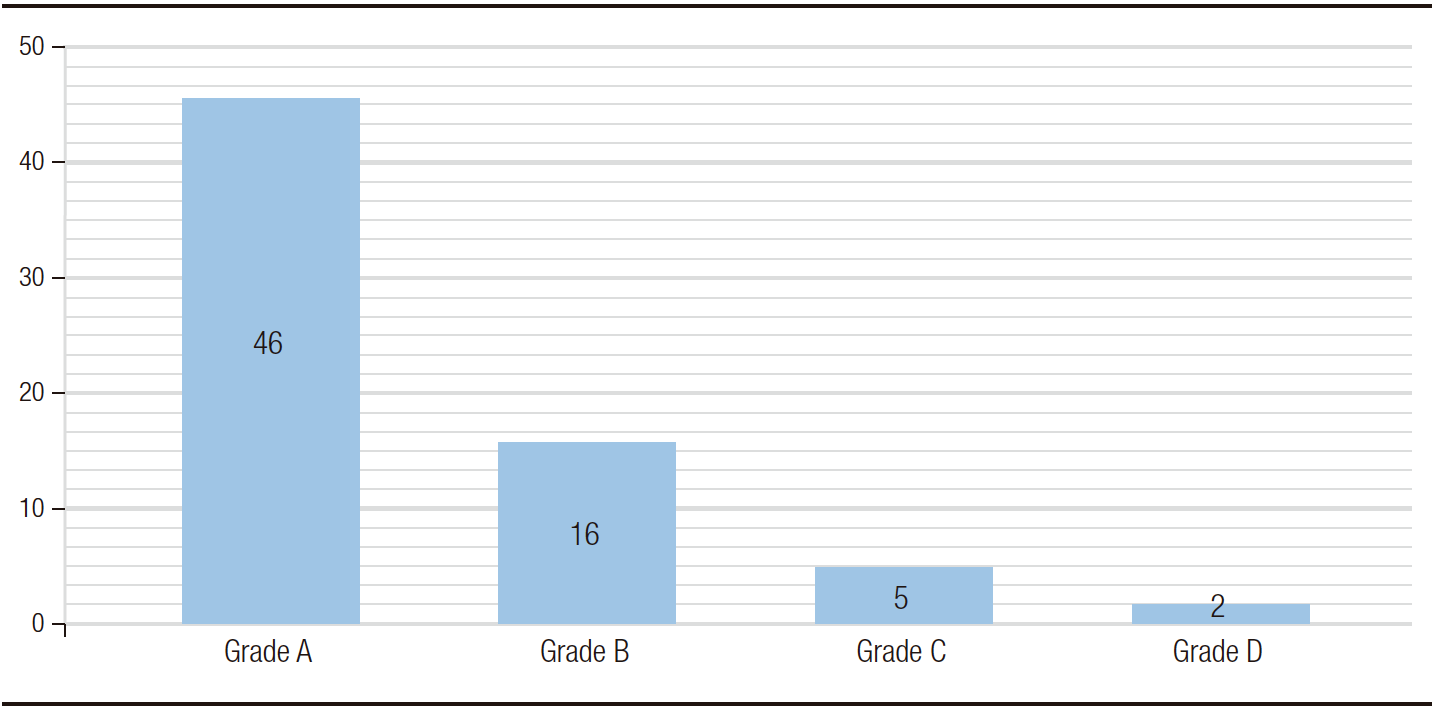
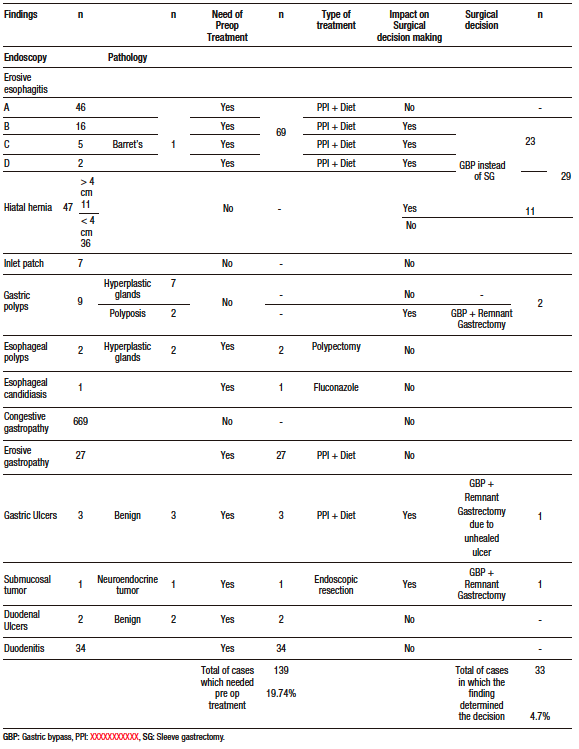
The prevalence of erosive esophagitis was 9.8% (n: 69). Grade A was the most frequent esophagitis with 46 cases (6.5%), followed by grade B with 16 cases (2.3%), grade C with 5 cases (0.7%) and D with 2 cases (0.3%) (Figure 1). Suspected Barret’s esophagus (BE) was described endoscopically in 5 patients, of which only one (0.1%) was confirmed by pathological anatomy. The remaining 4 patients were described as gastric metaplasia. All patients with suspected BE also had some grade of esophagitis. Patients with esophagitis were treated with double dose proton pump inhibitors (PPI) for at least two weeks before surgery. Forty-seven patients (6.7%) were endoscopically diagnosed with hiatal hernia, of whom 11 had a big hernia (> 4cm). Nineteen patients (40.4%) with hiatal hernia also had esophagitis, 5 patients (10.6%) of grade B or higher. In 29 patients (18 with esophagitis grade B or higher, 6 with 4 cm hiatal hernia or higher and 5 with both diagnoses) the esophageal endoscopic finding determined the surgical technique (gastric bypass instead of sleeve gastrectomy). The least frequent findings were inlet patch (gastric heterotopia in cervical esophagus) in 7 cases, esophageal polyps in 2 cases, and candidiasic esophagitis in 1 case.
Figure 1. Esophagitis (Los Angeles classification)
In the stomach, the most frequently observed finding was congestive gastropathy in 669 cases (95%), followed by erosive gastropathy in 27 cases (3.8%). Patients with erosive gastropathy were treated with double dose of PPI for at least two weeks before surgery. Peptic ulcers were detected in 3 patients who were treated with PPI for 3 months. A follow-up endoscopy was performed after treatment. In one of the patients there was no resolution of the ulcer, hence it was decided to perform resective gastric bypass (with gastrectomy of the gastric remnant). Gastric polyps were found in 9 patients, 7 of which underwent endoscopic polypectomy and were described in the anatomopathological study as hyperplastic or fundic gland polyps. Two of the cases were reported as gastric polyposis (more than 50 polyps). It was decided to perform a gastric bypass with resection of the remnant stomach in both cases due to the impossibility of endoscopic access to the excluded stomach after bypass. A fourth gastric bypass with resection of the remnant stomach was performed in a patient whose preoperative endoscopy revealed a submucosal lesion that was resected endoscopically. Pathological analysis with immunohistochemistry showed a well-differentiated grade 1 neuroendocrine tumor.
Duodenitis was identified in 34 patients (4.8%) who were treated with double dose of PPI for at least two weeks before surgery. Duodenal ulcers were found in two patients (0.3%). Medical treatment was performed with resolution of the lesions in the endoscopic follow-up performed at three months. In both patients, a RYGB was performed. We considered that the finding did not condition the surgical technique due to the good response to treatment and the low incidence of duodenal ulcers after gastric bypass. No villous atrophy was found in any patient.
One hundred forty-eight patients (21%) tested positive for Helicobacter pylori infection. All patients diagnosed with ulcers, either gastric or duodenal, had infection by the bacterium. Positive patients were treated with triple therapy (dexlansoprazole 60 mg BID, amoxicillin 1000 mg BID, and clarithromycin 500 mg BID) for 14 days. Stool antigen testing was performed 4 weeks after the end of treatment. The eradication rate was 83.1% (n:123). Patients who tested positive after therapy were prescribed a two-week course of metronidazole (500 mg BID) plus clarithromycin.
No significant differences were found in the prevalence of endoscopic findings and Helicobacter pylori according to sex (p 1, p 0.76), age (p 0.43, p 0.84) and body mass index (BMI) (p 0.36, p 0.12). There was no statistical difference between the groups.
It was observed that 19.7% (n: 139) of endoscopic findings needed a preoperative medical treatment and in 4.7% (n: 33) of the cases the surgical decision was determined by the endoscopic finding (Table 1). This means that in 24.4% of the endoscopies a treatable disease or a decisive finding was found.
Table 1. Endoscopic findings, the need of preoperative treatment and the impact on surgical decision
Discussion
Upper gastrointestinal endoscopy as a routine study prior to bariatric surgery continues to be subject of debate worldwide. A survey was published in November 20228 whose objective was to identify whether surgeons requested preoperative upper gastrointestinal endoscopy to bariatric surgery patients. A total of 121 surgeons were surveyed and only 53.7% responded that they routinely perform preoperative upper gastrointestinal endoscopy. In a 2014 UK survey conducted by the British Obesity and Metabolic Surgery Society (BOMSS), 90% of UK bariatric units surveyed included preoperative upper gastrointestinal endoscopy routinely or selectively.9
The preoperative diagnosis of certain pathologies would condition the type of surgical technique to be performed. Such is the clear example of patients diagnosed with hiatal hernia with gastroesophageal reflux, in whom the performance of a gastric sleeve without repairing the hernia could worsen their reflux disease. Another clear example is the diagnosis of polyps or ulcers with pathological anatomy predictive of malignancy, in which the literature recommends the gastric sleeve to be able to endoscopically control that stomach in the future, or the resective gastric bypass (with gastrectomy of the excluded stomach), thus avoiding the formation of future cancerous lesions that cannot be detected.
In 2020, the International Federation for Surgery of Obesity and Metabolic Disorders (IFSO) recommended the routine performance of upper gastrointestinal endoscopy in all patients who are candidates for bariatric surgery, since it was observed that in 25.3% of asymptomatic patients there could be findings that modify behavior or even contraindicate surgery, especially if the patient underwent some type of gastric bypass.10 The European Association for Endoscopic Surgery also recommends upper gastrointestinal videoendoscopy for all bariatric patients, with even more emphasis on those in whom a RYGB will be performed.11 In contrast, the American Association for Gastrointestinal Endoscopy recommends endoscopy only for symptomatic patients scheduled for bariatric surgery.12
According to the literature, the most prevalent preoperative endoscopic finding is the presence of inflammatory gastropathies. Mizelle D’Silva found 46.2% of patients with gastritis and 13.2% with gastric erosions.13 In our series congestive gastropathy was the most frequent finding (95%), and we found erosive gastropathy in 3.8% of the patients.
BE deserves important consideration in the management of bariatric surgical patients. In individual studies, BE was found in < 1% to 15% of preoperative endoscopy.6,14-16 Performing a sleeve gastrectomy in patients with BE may worsen this condition. There are even reports of increased incidence of de novo Barrett’s after sleeve gastrectomy. In this regard, RYGB is associated with a possible improvement of gastroesophageal reflux disease.17-19 In our report, BE was suspected endoscopically in 5 patients (0.7%). Saarinen et al. examined the endoscopic findings of 1275 bariatric patients and reported a BE rate of 3.7%.20 In a meta-analysis of 29 studies, involving 13434 patients, the prevalence of BE was 0.9%.21 Our approach to this diagnosis was to perform a RYBP in patients with endoscopic suspicion of BE.
Helicobacter pylori infection causes gastric barrier dysfunction, a process that increases paracellular permeability and allows other bacteria to translocate between gastric mucosal cells.22 For this reason, it is considered the leading cause of peptic ulcer disease. Therefore, multiple clinical guidelines call for Helicobacter pylori to be tested for and, if present, eradicated in all patients with peptic ulcers.23 In favor of eradication in patients candidates to bariatric surgery is the fact that it decreases the risk of peptic lesions in the gastrojejunoanastomosis area after gastric bypass, which could reduce early and late postoperative symptoms and complications. Schirmer et al.24 analyzed 560 patients, 206 of whom were tested for Helicobacter pylori infection, 62 (30.1%) of whom were positive and received treatment. Patients screened for Helicobacter pylori had a lower incidence of postoperative marginal ulcers (2.4%) than patients who were not screened (6.8%). Preoperative endoscopy makes it possible, through biopsy, to detect this microorganism in the anatomopathological study. In our group, this finding was observed in 147 (21%) patients, 3 of whom had peptic ulcer and 4 duodenal ulcer. Abdallah et al.25found Helicobacter pylori infection in 98 of the 643 patients studied (15.2%) and only 3 patients had concurrent peptic ulcer. Mizelle D’Silva et al.13 found Helicobacter pylori in 46.7% of
675 patients analyzed. In a cross-sectional study of 356 patients in Saudi Arabia, the prevalence of Helicobacter pylori was 41%.26
Multiple studies support the association between obesity and neoplastic diseases. Calle et al.27 after analyzing a population of more than 900000 American adults, found that 57145 cancer deaths occurred during 16 years of follow-up. In both men and women, BMI was significantly associated with higher rates of cancer death. Engeland et al.28 reported 2245 cases of esophageal cancer in Norway. This study did not control for smoking, alcohol intake, or diet, but reported that obese men had a 2.58-fold higher risk of death from esophageal adenocarcinoma than men of normal weight. Moulla et al.6 in an endoscopic analysis of 636 obese patients, found 4 patients (0.7%) with malignant neoplasms of the upper gastrointestinal tract. In this regard, in our series, one patient was diagnosed with a well-differentiated grade 1 neuroendocrine tumor at preoperative endoscopy and this finding resulted in a modification of the surgical approach.
As mentioned above, preoperative endoscopy can reveal numerous pathologies of the upper gastrointestinal tract that, sometimes, are not suspected by clinical evaluation of the patient alone, which can change the surgical approach. Moulla et al. 29 observed that a change of surgical strategy had to be performed due to pathologic endoscopic findings in 10 of 636 cases (1.6%). They recommend that preoperative upper gastrointestinal endoscopy should be considered in all obese patients before bariatric procedure.6 D’Silva et al.13 concluded that esophagogastroduodenoscopy should be mandatory as a preoperative investigation in all bariatric surgery patients, as its preoperative findings resulted in a change of the planned surgical procedure in 67 (9.9%) patients. In our report in 4.7% of the cases the surgical decision was determined by the endoscopic finding. Therefore, we conclude that endoscopic findings do have an impact on the choice of surgical technique, agreeing with Moulla and D’Silva et al.
Like us, Frigg et al. recommend routine endoscopy before bariatric surgery due to the high prevalence of upper gastrointestinal lesions and the influence they have on the surgical approach to be followed.29
In contrast, Bennett et al.5 evaluated 48 clinical trials with 12261 patients in a systematic review and concluded that preoperative endoscopy should be considered optional in asymptomatic patients. They observed that 7.8% of the endoscopic findings resulted in a change in surgical treatment. After eliminating benign findings such as hiatal hernia, gastritis and peptic ulcer, the percentage dropped to 0.4%. They also observed changes in medical treatment in 27.5% of cases.
Conclusion
Given the high relationship between obesity and diseases of the upper gastrointestinal tract, and the repercussion that these diseases can have in the postoperative period of a bariatric patient, we consider that upper gastrointestinal endoscopy represents a valuable tool in the preparation of the patient who is a candidate for bariatric surgery.
It could give an important information in almost one third of patients, as well as show us the need of a preoperative additional treatment or a change on surgical decision.
Upper gastrointestinal endoscopy should be taken into account in the presurgical evaluation of all patients candidates to bariatric surgery regardless of sex, age or BMI.
Consent for Publication. Anonymized data were used for the elaboration of this article, which did not distort its scientific value.
Intellectual Property. The authors declare that the data, figures and tables presented in the manuscript are original and were carried out at their belonging institution.
Funding. The authors declare that there were no external sources of funding.
Conflict of interest. The authors declare that they have no conflicts of interest in relation to this article.
Copyright
© 2023 Acta Gastroenterológica latinoamericana. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license, which allows non-commercial use, distribution, and reproduction, provided the original author and source are acknowledged.
Cite this article as: Airaldi N, García M, Gigena A R et al. Endoscopic Findings in Candidate Patients for Bariatric Surgery. Acta Gastroenterol Latinoam. 2023;53(4):361-368. https://doi.org/10.52787/agl.v53i4.360
References
- World Health Organization (2019). International statistical classification of diseases and related health problems (11th ed.).
- The International Federation for the Surgery of Obesity and Metabolic Disorders. 6th IFSO Global Registry Report 2019. IFSO 2021.
- Manish Parikh. Preoperative Endoscopy Prior to Bariatric Surgery: A Systematic Review and Meta-Analysis of the Literature. Obes Surg. 2016(12);26:2961-6.
- Bravo-Torreblanca, Carlos et al. Correlation of pre and postoperative endoscopic findings in patients subjected to bariatric surgery. Cir. gen [online].2013, vol.35, n.1, pp.20-24. ISSN 1405-0099.
- Bennett S, Gostimir M, Shorr R, et al. The role of routine preoperative upper endoscopy in bariatric surgery: a systematic review and meta-analysis. Surg Obes Relat Dis. 2016 Jun;12(5):1116-1125. DOI: 10.1016/j.soard.2016.04.012. Epub 2016 Apr 14. PMID: 27320221.
- Moulla Y, Lyros O, Mehdorn M, et al. Preoperative Upper-GI Endoscopy Prior to Bariatric Surgery: Essential or Optional? Obes Surg. 2020 Jun;30(6):2076-2084. DOI: 10.1007/s11695-020-04485-5
- Mangeaud A, Elías Panigo DH. 2018 R-Medic. R-Medic. A simple and intuitive statistical analysis software. Methodo 2018 Mar;3(1):18-22
- Quake SYL, Mohammadi-Zaniani G, Musbahi A, et al. Routine Use of Esophago-gastro-duodenoscopy (EGD) in Bariatric Surgery-an International Survey of Our Current Practice. Obes Surg. 2022 Nov;32(11):3627-3634. DOI: 10.1007/s11695-022-06252-0. Epub 2022 Sep 3. PMID: 36057022; PMCID: PMC9440328.
- Zanotti D, Elkalaawy M, Hashemi M, et al. Current status of preoperative oesophago-gastro-duodenoscopy (OGD) in bariatric NHS units-a BOMSS survey. Obes Surg. 2016;26(9):2257-62
- Brown WA, Johari Halim Shah Y, Balalis G, et al. IFSO position statement on the role of esophago-gastro-duodenal endoscopy prior to and after bariatric and metabolic surgery procedures. Obes Surg. 2020;30(8):3135–53.
- Sauerland S, Angrisani L, Belachew M, et al. Obesity surgery: evidence-based guidelines of the European Association for Endoscopic Surgery (EAES) Surg Endosc. 2005;19:200-221.
- Anderson MA, Gan SI, Fanelli RD, et al. Role of endoscopy in the bariatric surgery patient. Gastrointest Endosc. 2008;68:1-10.
- D’Silva M, Bhasker AG, Kantharia NS, et al. High-Percentage Pathological Findings in Obese Patients Suggest that Esophago-gastro-duodenoscopy Should Be Made Mandatory Prior to Bariatric Surgery. Obes Surg. 2018 Sep;28(9):2753-2759. DOI: 10.1007/s11695-018-3230-z. PMID: 29681019.
- Parikh M, Liu J, Vieira D, et al. Preoperative Endoscopy Prior to Bariatric Surgery: a Systematic Review and Meta-Analysis of the Literature. Obes Surg. 2016 Dec;26(12):2961-2966. DOI: 10.1007/s11695-016-2232-y. PMID: 27198238.
- Wolter S, Duprée A, Miro J, et al. Upper Gastrointestinal Endoscopy prior to Bariatric Surgery-Mandatory or Expendable? An Analysis of 801 Cases. Obes Surg. 2017 Aug;27(8):1938-1943. DOI: 10.1007/s11695-017-2622-9. PMID: 28243860.
- Ooi GJ, Browning A, Hii MW, et al. Perioperative screening, management, and surveillance of Barrett’s esophagus in bariatric surgical patients. Ann N Y Acad Sci. 2020;1481(1):224-235.
- Sebastianelli L, Benois M, Vanbiervliet G, et al. Systematic endoscopy 5 years after sleeve gastrectomy results in a high rate of Barrett’s esophagus: results of a multicenter study. Obes Surg 2019;29(5):1462-9.
- Braghetto I, Korn O. Late esophagogastric anatomic and functional changes after sleeve gastrectomy and its clinical consequences with regards to gastroesophageal reflux disease. Dis Esophagus 2019;32(6):doz020
- Felsenreich DM, Ladinig LM, Beckerhinn P, et al. Update: 10 years of sleeve gastrectomy-the first 103 patients. Obes Surg 2018;28(11):3586-94.
- Saarinen T, Kettunen U, Pietiläinen KH, et al. Is preoperative gastroscopy necessary before sleeve gastrectomy and Roux-en-Y gastric bypass? Surg Obes Relat Dis. 2018 Jun;14(6):757-762. DOI: 10.1016/j.soard.2018.01.021. Epub 2018 Feb 14. PMID: 29477376.
- Qumseya B, Gendy S, Wallace A, et al. Prevalence of Barrett’s esophagus in obese patients undergoing pre-bariatric surgery evaluation: a systematic review and meta-analysis. Endoscopy. 2020 Jul;52(7):537-547. DOI: 10.1055/a-1145-3500. Epub 2020 Apr 23. Erratum in: Endoscopy. 2020 May 18: PMID: 32325514.
- Buti L, Ruiz-Puig C, Sangberg D, et al. CagA-ASPP2 complex mediates loss of cell polarity and favors H. pylori colonization of human gastric organoids. Proc Natl Acad Sci U S A 2020;117(5):2645–55.
- NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH consensus development panel on helicobacter pylori in peptic ulcer disease. JAMA 1994;272(1):65-9.
- Schirmer B, Erenoglu C, Miller A. Flexible endoscopy in the management of patients undergoing Roux-en-Y gastric bypass. Obes Surg. 2002;12:634-8.
- Abdallah H, El Skalli M, Mcheimeche H, et al. Indications for upper gastrointestinal endoscopy before bariatric surgery: a multicenter study. Surg Endosc. 2023 Feb;37(2):1342-1348.
- Al Eid A, Al Balkhi A, Hummedi A, et al. The utility of esophagogastroduodenoscopy and Helicobacter pylori screening in the preoperative assessment of patients undergoing bariatric surgery: A cross-sectional, single-center study in Saudi Arabia. Saudi J Gastroenterol. 2020 Jan-Feb;26(1):32-38. DOI: 10.4103/sjg.SJG_165_19. PMID: 31898643; PMCID: PMC7045771.
- Calle EE, Rodriguez C, Walker-Thurmund K, et al. Overweight, obesity and mortality from cancer in a prospectively studied cohort of US adults. N Eng J Med 2003;348(17):1625-38.
- Engeland A, Tretli S, Bjørge T. Height and body mass index in relation to esophageal cancer; 23-year follow-up of two million Norwegian men and women. Cancer Causes Control. 2004 Oct;15(8):837-43. DOI: 10.1023/B:CACO.0000043434.21558.ea. PMID: 15456997.
- Frigg A, Peterli R, Zynamon A, et al. Radiologic and endoscopic evaluation for laparoscopic adjustable gastric banding: preoperative and follow-up. Obes Surg. 2001 Oct;11(5):594-9.
Correspondencia: Manuel García
Email: drmangarcia@gmail.com
Acta Gastroenterol Latinoam 2023;53(4)361-368
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE