Bleenis Gutiérrez Castro ID· Jóse Luis Fernández ID· Federico Cassella ID· Andrés Wonaga ID
Luis Viola ID
Centro Integral de Gastroenterología.
Ciudad Autónoma de Buenos Aires, Argentina.
Acta Gastroenterol Latinoam 2023;53(1):59-67
Received: 16/01/2023 / Accepted: 17/03/2023 / Published online 30/03/2023 / https://doi.org/10.52787/agl.v53i1.292
Summary
Introduction. It is uncertain whether starting screening at 45 years old would improve colorectal cancer prevention and currently available studies in adults younger than 50 years old are limited. Aim. To evaluate the adenoma detection rate at different age intervals. Materials and methods. Colonoscopies performed on adult outpatients were analyzed. Adenoma detection was recorded in the total population and in patients with screening indication. First, patients were divided into two groups: 50 years or older (group A) and younger than 50 years (group B). Then, we analyzed the different age segments: up to 44 years (group 1) 45 to 49 (group 2), 50 to 54 (group 3), and 55 or older (group 4). Results. A total of 5090 patients were included, 2877 with indication for screening. Patients were divided as follows: 3883 in group A, 1207 in group B, 811 in group 1, 396 in group 2, 749 in group 3 and 3134 in group 4. In the total population, adenoma detection was 20.5%: 23.5% in group A, 10.5% in group B (p = 0.000); 8.3% in group 1, 14.8% in group 2, 18.1% in group 3, and 24.8% in group 4 (group 1 vs. group 2: p = 0.001, group 2 vs. group 3: p = 0.189, and group 3 vs. group 4: p = 0.000). In the screening population, adenoma detection was 20.5%: 21.4% in group A, 14.8% in group B (p = 0.004); 13.1% in group 1, 17.0% in group 2, 16.1% in group 3, and 22.8% in group 4 (group 1 vs. group 2: p = 0.31; group 2 vs. group 3: p = 0.81; and group 3 vs. group 4: p = 0.001). Conclusion. Adenoma detection is not different between 45 to 49 and 50 to 54 years of age, and is lower below 45 years of age, which suggest starting colorectal cancer screening at this age.
Keywords. Adenoma detection rate, screening, colorectal cancer.
La tasa de detección de adenomas en diferentes intervalos de edad sugiere iniciar el cribado de cáncer colorrectal a los 45 años
Resumen
Introducción. Es incierto si comenzar el cribado a los 45 años mejoraría la prevención del cáncer colorrectal y los estudios actualmente disponibles en adultos menores de 50 años son limitados. Objetivo. Evaluar la tasa de detección de adenomas en diferentes intervalos de edad. Material y métodos. Se analizaron las colonoscopias realizadas a pacientes adultos ambulatorios. La detección de adenomas se registró en la población total y en pacientes con indicación de cribado. Primero, los pacientes se dividieron en dos grupos: 50 años o más (grupo A) y menores de 50 años (grupo B). Luego, analizamos los diferentes segmentos de edad: hasta 44 años (grupo 1), 45 a 49 (grupo 2), 50 a 54 (grupo 3), y 55 o más (grupo 4). Resultados. Se incluyeron 5090 pacientes, 2877 con indicación de cribado. Los pacientes se dividieron de la siguiente manera: 3883 en el grupo A, 1207 en el grupo B, 811 en el grupo 1, 396 en el grupo 2, 749 en el grupo 3 y 3134 en el grupo 4. En la población total, la detección de adenomas fue del 20,5 %: 23,5% en el grupo A, 10,5% en el grupo B (p = 0,000); 8,3% en el grupo 1, 14,8% en el grupo 2, 18,1% en el grupo 3 y 24,8% en el grupo 4 (grupo 1 vs. grupo 2: p = 0,001, grupo 2 vs. grupo 3: p = 0,189, y grupo 3 vs. grupo 4: p = 0·000). En la población de cribado, la detección de adenomas fue del 20,5 %: 21,4 % en el grupo A, 14,8 % en el grupo B (p = 0,004); 13,1 % en el grupo 1, 17,0 % en el grupo 2, 16,1 % en el grupo 3 y 22,8 % en el grupo 4 (grupo 1 vs. grupo 2: p = 0,31; grupo 2 vs. grupo 3: p = 0,81 y grupo 3 vs grupo 4: p = 0,001). Conclusión. La detección de adenomas no es diferente entre los 45 a 49 y los 50 a 54 años, y es menor por debajo de los 45 años, lo que sugiere iniciar el cribado de cáncer colorrectal a esta edad.
Palabras claves. Detección de adenomas, screening, cáncer colorrectal.
Abbreviations
CRC: Colorectal cancer.
ADR: Adenoma detection rate.
HR: High-risk.
RC: Right colon.
Introduction
Colorectal cancer (CRC) is the third most frequent cancer and the second leading cause of cancer death worldwide.1 In the United States, CRC is the fourth most commonly diagnosed cancer, resulting in 50,000 deaths per year.2 Although CRC remains a leading cause of cancer, declining trends in incidence and mortality have been reported in Europe and the United States.3 These trends have been attributed primarily to increased screening policies and the resulting availability of improved therapies.2, 4
Colonoscopy has become the preferred screening method for the detection and removal of precancerous lesions, and the reduction in CRC incidence and mortality with this strategy has been largely demonstrated over the past decade.5 The importance of adenoma detection in reducing the risk of interval colorectal cancer, advanced stage interval cancer and fatal interval cancer was also demonstrated, for both male and female patients and for both proximal and distal CRC.4 Thus, the adenoma detection rate (ADR) became a relevant indicator of colonoscopy quality and professional societies recommended that colonoscopists include ADR in their performance measurement.6, 7 Adenomas are the precursors of perhaps 70% of CRC cases, and this fact is particularly important when considering advanced adenomas (size equal to or greater than 1 cm, villous components or high-grade dysplasia).6 In a large retrospective study, a 3% reduction in CRC mortality was observed for every 1% increase in ADR.2
In recent years, some issues related to age and risk of CRC have emerged. In fact, 10% to 12% of all CRCs are diagnosed in patients younger than 50 years, and these cancers tend to be more advanced and devastating at presentation.8, 9 Several biological and environmental risk factors have been associated with this trend, such as hereditary conditions, dietary patterns, obesity, diabetes, physical activity, early exposure to antibiotics, smoking, alcohol and processed meat consumption. However, whether modifiable or non-modifiable risk factors, or both, explain the increased incidence of early-onset CRC remains to be determined, and a better understanding of these factors is needed to incorporate them into preventive strategies that include individuals younger than 50 years in the screening group.10
Despite the observed increase in CRC incidence in persons younger than 50 years of agein several high-income countries,8, 11 most consensus statements maintained the recommendation to begin CRC screening at age 50 in persons at medium -risk, except for African Americans. This is based on the fact that there was insufficient evidence to recommend screening in asymptomatic persons younger than 50 years.9, 11, 12 Recently, the American Cancer Society recommended lowering the starting age for CRC screening from 50 to 45 years.2, 13 However, currently available studies in younger adults are limited, and it remains uncertain whether earlier screening would improve CRC prevention.14, 15 Siegel and colleagues observed thatcurrently available studies did not examine the temporal patterns by age, calendar period and year of birth. They developed a model to understand disease trends, they found variations in CRC incidence patterns by age, tumor subsite, calendar period, and birth cohort, with an increased incidence of CRC in young adults, and suggested reconsidering the initiation of CRC screening before age 50.3
As a contribution to the search for an answer to this complex issue, we set out in this study to evaluate ADR in different age groups, with special attention to the age groups around 50 years.
Materials and Methods
Study Design
An exploratory and retrospective review of colonoscopies performed at our center between January 2016 and December 2018 was performed. Our center is a private institution located in Buenos Aires, Argentina, dedicated to the clinical practice of gastroenterology and endoscopy.
Outpatients over 18 years of age who underwent a colonoscopy for any indication were included. Virtually all patients attending the center belonged to private health insurance systems and lived in the metropolitan area of Buenos Aires, and most of them were Caucasian. Patients with history of colon surgery, inflammatory bowel disease, or personal CRC were excluded from the study. Patients in whom biopsy results were not available were also excluded. If two or more colonoscopies were performed on the same patient during the study period, only one was included (polyp/adenoma found).
Data Analysis
We reviewed the written report of each study and the corresponding histopathologic inform. Based on histopathogical criteria, ADR was considered as the percentage of patients with at least one adenoma, considering the more severe lesion and independent of the number, location or size of lesions found. Assessing the detection rate of neoplasms, overall detection and detection of high-risk adenomas, carcinomas and adenomas of the right colon were analyzed.
Patient and study characteristics were recorded in a specific software: age, sex, indication for colonoscopy, cecal intubation rate, and Boston scale.16 Polyps were evaluated according to number, location, size, and histopathologic findings.
First, patients were divided by age in two groups: 50 years or older (group A) and younger than 50 years (group B). Then, we analyzed the different subsegments: up to 44 years (group 1), 45 to 49 years (group 2), 50 to 54 years (group 3), and 55 or older (group 4). ADR was evaluated in each group, considering the total population as well as the screening population, which was defined as patients at low or medium risk of CRC or with a family history of CRC (high risk). Patients under surveillance for adenoma history and symptomatic patients completed the total population.
Colonoscopy Procedure
Colonoscopies were performed on consecutive outpatients under routine indications of daily clinical practice by five experienced endoscopists from our center, all with more than 10 years of practice (ADR greater than 25%).7 They used GIF-0140, GIF-0150 and GIF-0160 video endoscopes (Olympus Medical Systems Corp, Tokyo, Japan). All the procedures were performed under propofol sedation.
Patients were instructed to follow a diet excluding fruits, vegetables, cereals, seeds, and dairy products for 3 days before the procedure. Monosodium phosphate/disodium phosphate or sodium picosulfate were the selected cleansing agents, except for patients 70 years of age or older or with a history of kidney failure, severe hypertension or congestive heart failure. In these cases, polyethylene glycol was the recommended option. All bowel preparations were performed using a two-dose schedule.
Quality indicators for colonoscopy were colon preparation according to the Boston scale (with a cut-off of 6 points for adequate preparation with at least 2 points for each of the evaluated colon segments) and cecal intubation rate (as deep intubation into the cecum, with the tip of the endoscope being able to touch the appendiceal orifice, thus allowing visualization of the inner wall).7
Histopathology
Only adenomatous polyps were considered, including tubular, villous or serrated adenomas, and carcinomas. Hyperplastic polyps were excluded. High-risk (HR) adenomas were defined as those either larger than 1 cm (measured with an open biopsy forceps), villous or tubulo-villous, serrated, or with high-grade dysplasia.
Statistical Analysis
For the comparative statistical analysis of baseline characteristics, we used a chi-squared test for categorical variables and an unpaired Student’s t test for the continuous variables after confirming the normal distribution of our data. Comparisons of ADRs between age groups were performed using a chi-squared test. A P value of less than 0.05 was considered significant.
Ethical Aspects
Written informed consent for endoscopy was obtained from all the patients. No additional procedures were performed other than those indicated by the prescribing physicians or by the endoscopic findings. Patient information was mantained anonymous in accordance with local data protection regulations.
Our institutional reviewers considered that because we conducted a retrospective study using data from our endoscopy and pathology records, approval from an independent ethics committee was not necessary.
Funding
There was no source of funding.
Results
General data
We analyzed data from 6293 colonoscopies and we excluded 1203 studies because they did not meet the inclusion criteria or because they were performed on the same patient. We finally included 5090 patients. These patients were divided according to age as follows: 3883 were 50 years or older (group A), 1207 were younger than 50 years (group B), 811 were less than 45 years (group 1), 396 were between 45 and 49 years (group 2), 749 between 50 and 54 years (group 3), and 3134 were 55 or older (group 4).
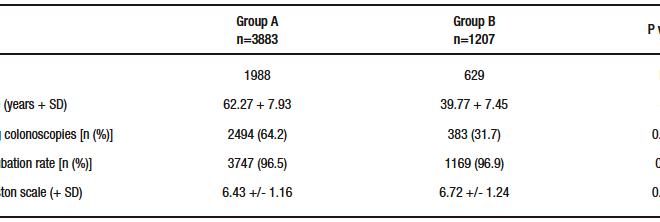
Of the 5090 patients, 2617 were women and 2473 were men. Their mean age was 56.91 + 12.38 years. The indication for colonoscopy was CRC screening in 2877 cases, surveillance for history of adenoma in 719 cases, and intestinal symptoms in 1494 cases. The cecal intubation rate was 96.6% and the mean Boston scale score was 6.50 + 1.19. The distribution of these findings in groups 1 and 2 is shown in Table 1. There was no significant difference in sex: 424 women of 811 patients in group 1 (52.3%), 205 of 396 in group 2 (51.8%), 364 of 749 in group 3 (48.6%), and 1624 of 3134 in group 4 (51.8%) (1 vs. 2: p = 0.92, 2 vs. 3: p = 0.34, 3 vs. 4: p = 0.12).
Table 1. Comparison between patients older than 50 years (Group A) and younger than 50 years (Group B)
Total Population
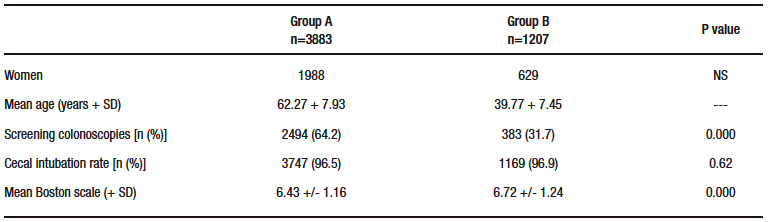
In the total population, 1043 adenomas were detected (ADR 20.5%), 916 of them in group A (ADR 23.5%) and 127 in group B (ADR 10.5%) (p = 0,000) (Figure 1). According to the distribution in different age segments, 68 adenomas were found in group 1 (ADR 8.3%), 59 in group 2 (ADR 14.8%), 136 in group 3 (ADR 18.1%), and 780 in group 4 (ADR 24.8%). The comparison between these groups showed: group 1 vs. group 2: p = 0.001; group 2 vs. group 3: p = 0.189; and group 3 vs. group 4: p = 0.000 (Figure 2). Adenomas were detected in 433 women (16.5%) and 610 men (24.7%) (p = 0.000). In an additional analysis to avoid bias introduced by younger patients, we compared the subgroups of patients aged 40 to 44 years and 45 to 49 years. Adenomas were detected in 29 of 310 patients (9.3%) and 59 of 396 patients (14.8%), respectively (p = 0.04).
Figure 1. Adenoma detection rate in total population: patients at least 50 years old (Group A) and older than 50 years old (Group B)
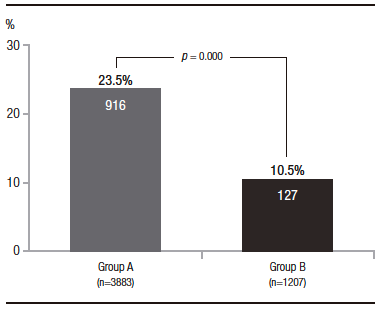
Figure 2. Adenoma detection rate in total population: patients younger than 45 years old (Group 1), 45 to 49 years old (Group 2), 50 to 54 years old (Group 3), and older than 54 years old (Group 4)
Screening Population
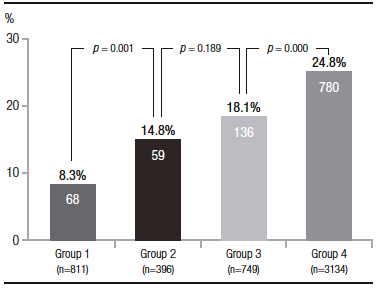
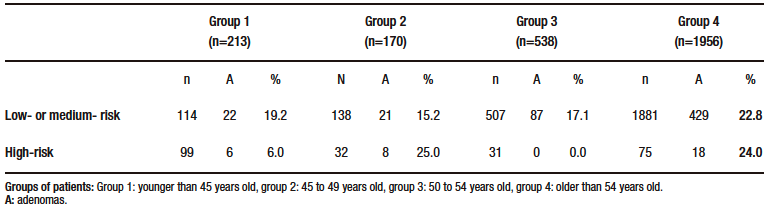
Of the 2877 patients who underwent screening colonoscopy, adenomas were detected in 591 patients (ADR 20.5%), including 534 in the 2494 patients in group A (ADR 21.4%) and 57 in the 383 patients in group B (ADR 14.8%) (p = 0.004). According to the distribution in different age segments, 28 adenomas were found in 213 patients in group 1 (ADR 13.1%), in 29 of 170 in group 2 (ADR 17.0%), in 87 of 538 in group 3 (ADR 16.1%), and in 447 of 1956 in group 4 (ADR 22.8%). The comparison between these groups showed: group 1 vs. group 2: p = 0.31, group 2 vs. group 3: p = 0.81; and group 3 vs. group 4: p = 0.001 (Table 2). The differences between patients at low or medium risk of CRC and patients with a family history of CRC are shown in Table 3.
Table 2. Adenoma detection in different settings
Table 3. Differences between low- or medium-risk and high-risk patients
High-Risk Adenoma Detection Rate
HR adenomas were detected in 520 patients of the total population (HR ADR 10.2%), 453 of them in group A (HR ADR 11.7%) and 67 in group B (HR ADR 5.6%) (p = 0.000). According to the distribution in different age segments, 31 adenomas were found in 811 patients in group 1 (HR ADR 3.8%), in 36 of 396 patients in group 2 (HR ADR 9.1%), in 66 of 749 patients in group 3 (HR ADR 8.8%) and in 387 of 3134 patients in group 4 (HR ADR 12.3%). The comparison between these groups showed: group 1 vs. group 2: p = 0.000, group 2 vs. group 3: p = 0.96; and group 3 vs. group 4: p = 0.008 (Table 2).
Adenoma Detection Rate in the Right Colon
Right colon (RC) adenomas were detected in 481 patients of the total population (RC ADR 9.4%), 425 of them in group A (RC ADR 10.9%) and 56 in group B (RC ADR 4.6%) (p = 0.000). According to the distribution in different age segments, 26 adenomas were found in 811 patients in group 1 (RC ADR 3.2%), in 30 of 396 in group 2 (RC ADR 7.5%), in 53 of 749 in group 3 (RC ADR 7.0%), and 372 of 3134 in group 4 (RC ADR 11.8%). The comparison between these groups showed: group 1 vs. group 2: p = 0.002, group 2 vs. group 3: p = 0.85; and group 3 vs. group 4: p = 0.000 (Table 2).
Colorectal Cancer
We found CRC in 34 patients in the total population (0.67%), 13 of them during a screening colonoscopy (0.45%) and 21 in the non-screening studies (0.95%) (p = 0.047). Twenty-nine patients (0.75%) with CRC belonged to group A and 5 to group B (0·41%) (p = 0.31). Four patients belonged to group 1, 1 to group 2, 4 to group 3, and 25 to group 4 (group 2 vs. group 3: p = 0.66, group 3 vs. group 4: p = 0.64).
Discussion
The main finding of our study is that there are no significant differences in the ADR between the ages of 45 to 49 years and 50 to 54 years (Figure 2). This finding is consistent with our previous report in a smaller population and could support the recommendation to start colorectal cancer screening at 45 years of age.17 This observation was valid not only for the total population studied but also for patients who underwent screening colonoscopy, for HR ADR, and for ADR in the right colon.
We began our analysis by confirming that ADR is significantly higher in patients older than 50 years
(Figure 1), as previously observed in a large database, with an increase with each decade of life after age 50.18 This fact is well known and was the reason for the initiation of CRC screening starting at this age, with a strong evidence-based recommendation.2, 11 However, some epidemiological studies showed that CRC was increasing in younger populations and this was the first indication to suggest a change in this policy. Although a continued decline in CRC mortality rates was predicted in European countries thorugh 2018 and some reports did not confirm that ADR was increasing in younger people,19, 20 the incidence of CRC in adults younger than 55 years in the United States has doubled in two decades.3 A few years ago, some scientific societies in the United States began to support earlier colonoscopies, either in non-white individuals (American College of Gastroenterology and American Society of Gastrointestinal Endoscopy)11 or in selected younger populations, such as patients with family history of CRC or advanced adenomas (American College of Physicians and Institute for Clinical Systems Improvement).21, 22 Following this trend, Peterse and colleagues developed a predictive microsimulation analysis and concluded that the best efficacy rate was obtained by initiating colonoscopy screening at age 45 years.13 Based on these findings, the American Cancer Society recommended, as a qualified recommendation, lowering the starting age from 50 to 45 years for all medium risk individuals, with either a high-sensitivity stool-based test or a structural examination, depending on patient preference and test availability.2 In contrast, other American organizations (United States Preventive Service Task Force and United States Multi-Society Task Force of Colorectal Cancer) and European guidelines still recommend screening from age 50.23, 24
Given the insufficient evidence from population-based studies, the decision to initiate colonoscopy earlier remains controversial and should probably be limited to countries where an increase in the early-onset of CRC has been demonstrated.23 Some important concerns have been outlined to this strategy: the increased incidence of CRC in younger individuals had wide confidence intervals suggesting imprecision, the choice of a group between 45 and 49 years was arbitrary, the use of efficacy rate in the predictive model was considered insufficient to evaluate the cost-effectiveness, the cost of CRC screening should increase, and the compliance by patients and health care professionals could be a challenge.14, 15 Thus, considering that the detection and treatment of adenomas is a good prophylaxis for the development of CRC,5 we hypothesized that ADR analysis at different age intervals should contribute to determine when the risk of CRC increases. Surveillance, Epidemiology, and End Results (SEER) data showed that life years lost due to CRC were comparable at ages 45-49 years (5.1%) and 50-54 years (7.6%), and Syed and colleagues observed that the majority of the early-onset CRC group was aged 40 to 49 years (71.1%).2, 25
However, we found few studies that analyzed the relationship between ADR and age. In 2015, Hemmasi and colleagues compared patients of 40-49 years old with those aged 50-59 years in a small sample of 740 screening colonoscopies and found no statistical differences between the two groups (11.7% vs. 16.5%), but the age interval was too wide to draw any conclusions about age 45 years as a threshold for screening.26 Karsenti and colleagues were the first to examine ADR and HR ADR using age intervals of 5 years. In 6027 colonoscopies, their overall ADR was 28.6%, a figure higher than ours of 20.5% (24.8% when patients older than 54 years were included), with a similar proportion of patients younger than 45 years (16.9% vs. 15.9%).27 Our total ADR was similar to that observed in a large cohort study from Austria (19.7%).28 As in most studies, our ADR was significantly higher in male sex.
It is difficult to compare the population of Karsenti and colleagues and ours because the inclusion criteria were different. They included patients with a family or personal history of colonic polyps or CRC in the high-risk group, without providing explaining as to where symptomatic patients were included. For us, screening patients were both medium and high-risk (family history). Surveillance of adenomas and symptomatic cases were not considered screening, and patients with a personal history of CRC were excluded. Nevertheless, our results in the total population are consistent with those of Karsenti and colleagues, with a non-significant difference in ADR between the 45 to 49 age group and the 50 to 54 age group (21.2% vs. 25.2%).27 In a recent communication to Digestive Disease Week with an extremely high ADR, Dasari and colleagues found no differences between these groups (56.5% vs. 55.5%).29 Although our ADR in the 45-49 age group was lower than previously reported (21.2% to 56.5%), we also found this lack of difference.27, 29
Among other interesting data, we also observed that the difference in ADR was significantly lower in patients younger than 45 years and significantly higher in those older than 54 years. This observation was valid for the total population and for the subgroups studied, with the only exception of the difference at 45 years in the screening group (Table 2). Regarding the prevalence of CRC, it was very low in our population (0.67%) and significantly lower in the screening group. We could not obtain a statistically valid difference between the different age segments, due to the small number of cases.
The weakness of our study is the retrospective and single-center design (we do not know the family history of all patients). We did not detect a significant difference in ADR between ages below 45 years and 45 to 49 years in the screening population, which may be due to a type two error. In addition, we found little family history of CRC, while other authors noted that ADR was significantly higher in high-risk individuals 40 to 49 years (27.8% vs. 19.7%).30 Moreover, we cannot extrapolate our results to the community because of the single-center design nature of our study. In contrast, the homogeneity of our data was preserved by similar characteristics over the years, as the social status of the patients, the team (endoscopists, anesthesiologists and nurses) and the equipment used were the same. Another strength of the study is that it was conducted in a real-life scenario.
Conclusion
In conclusion, our ADR was not significantly different in patients aged 45 to 49 and 50 to 54 years and ADR was significantly lower in patients younger than 45 years old, suggesting a benefit in initiating CRC screening at this age. Direct evidence on the efficacy of screening in adults younger than 50 years is limited. Most large randomized controlled trials begin at this age, and trials demonstrating the benefit of age reduction are underpowered to perform age subgroup analyses.2 Therefore, larger prospective studies using an age interval-based approach should contribute to clarify this still controversial issue.3, 14.
Consent for Publication. Written informed consent was obtained from the patient or their parent, guardian, or relative to publish the data and/or clinical images for the benefit of science. A copy of the consent form is available to the editors of this journal.
Intellectual Property. The authors declare that the data, figures, and tables that appear in this article are original and were made in their belonging institutions.
Funding. The authors state that there were no external funding sources.
Conflict of Interest. The authors declare that he has no conflicts of interest in relation to this article.
Copyright
© 2023 Acta Gastroenterológica Latinoamericana. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license, which allows non-commercial use, distribution, and reproduction, provided the original author and source are acknowledged.
Cite this article as: Gutiérrez Castro B, Fernández J L, Cassella F et al. Adenoma Detection Rate at Different Age Intervals Suggests Starting Colorectal Cancer Screening at 45 Years of Age. Acta Gastroenterol Latinoam. 2023;53(1):59-67. https://doi.org/ 10.52787/agl.v53i1.292
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424.
- Corley D, Jensen C, Marks A. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298-306.
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst 2017;109:djw322.
- Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomized controlled trials and observational studies. BMJ 2014;348:g2467.
- Kaminski MF, Wieszczy P, Rupinski M, Wojciechowska U, Didkowska J, Kraszewska E, Kobiela J, Franczyk R, Rupinska M, Kocot B, Chaber-Ciopinska A, Pachlewski J, Polkowski M, Regula J. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology. 2017 Jul;153(1):98-105.
- Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT, Safdi MA, Faigel DO, Pike IM; ASGE/ACG Taskforce on Quality in Endoscopy. Quality indicators for colonoscopy. Am J Gastroenterol. 2006 Apr;101(4):873-85
- Viola LA, Cassella F, Wonaga A, Arnao Dellamea G, Di Paola L, Ubeira Salim R, Fernández JL. Implementation of a program to improve the quality of colonoscopy increases the neoplasia detection rate: a prospective study. Endosc Int Open. 2016 Jan;4(1):E68-72
- Maratt JK, Kahi CJ. Who is at risk for early-onset colorectal cancer? Clin Gastroenterol Hepatol 2020;2020;18:2686-8.
- Strum WB, Boland CR. Characterization and identification of colorectal cancer in persons younger than 50 years. Clin Gastroenterol Hepatol 2019;17:2600-2.
- Gausman V, Dornblaser D, Anand S, Hayes RB, O’Connell K, Du M, Liang PS. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin Gastroenterol Hepatol. 2020 Nov;18(12):2752-2759
- Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson DJ. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017 Jul;153(1):307-323.
- Siegel RL, Miller KD, Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970-2014. JAMA 2017;318:572-4.
- Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, Smith RA, Zauber AG, Lansdorp-Vogelaar I. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018 Jul 15;124(14):2964-2973.
- Anderson JC, Samadder NJ. To screen or not to screen adults 45-49 years of age: that is the question. Am J Gastroenterol 2018;113:1750-53.
- Imperiale TF, Kahi CL, Rex DK. Lowering the starting age for colorectal cancer screening to 45 years: who will come and should they? Clin Gastroenterol Hepatol 2018;16:1541-4.
- Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc 2010;72:686-92.
- Gutiérrez BR, Fernández J, Cassella F, Viola LA. Does the analysis of adenoma detection rate at different intervals of age support the recommendation to start colorectal cancer screening at 45 years old? (abstract). Gastrointest Endosc 2019;89 Suppl:AB404.
- Diamond SJ, Enestvedt BK, Jiang Z, Holub JL, Gupta M, Lieberman DA, Eisen GM. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest Endosc. 2011 Jul;74(1):135-40
- Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann Oncol. 2018 Apr 1;29(4):1016-1022
- Wonaga A, Gutiérrez B, Cassella F, Graciano J, Fernández JL, Viola LA. Is adenoma detection rate increasing in patients younger than age 50? (abstract). UEG J 2019;7:265-266.
- Qaseem A, Denberg TD, Hopkins RH Jr, Humphrey LL, Levine J, Sweet DE, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012 Mar 6;156(5):378-86.
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009 Mar;104(3):739-50.
- Mannucci A, Zuppardo RA, Rosati R, Di Leo M, Perea J, Cavestro GM. Colorectal cancer screening from 45 years of age: Thesis, antithesis and synthesis. World J Gastroenterol 2019;25:2565-80.
- Basu P, Ponti A, Anttila A, Ronco G, Senore C, Vale DB, Segnan N, Tomatis M, Soerjomataram I, Primic Žakelj M, Dillner J, Elfström KM, Lönnberg S, Sankaranarayanan R. Status of implementation and organization of cancer screening in The European Union Member States-Summary results from the second European screening report. Int J Cancer. 2018 Jan 1;142(1):44-56.
- Syed A, Abdul-Baki H, Tang A, Thakkar P, Farah K, Thakkar S. Colorectal cancer in young adults: identifying potential risks factors (abstract). Gastroenterology 2018;154 Suppl 1:S-777.
- Hemmasi G, Sohrabi M, Zamani F, Ajdarkosh H, Rakhshani N, Khoonsari M, Ameli M, Hatami K. Prevalence of colorectal adenoma in an average-risk population aged 40-50 versus 50-60 years. Eur J Cancer Prev. 2015 Sep;24(5):386-90.
- Karsenti D, Tharsis G, Burtin P, Venezia F, Tordjman G, Gillet A, Samama J, Nahon-Uzan K, Cattan P, Cavicchi M. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019 Jan 28;25(4):447-456.
- Ferlitsch M, Reinhart K, Pramhas S, Wiener C, Gal O, Bannert C, Hassler M, Kozbial K, Dunkler D, Trauner M, Weiss W. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA. 2011 Sep 28;306(12):1352-8.
- Dasari CS, Desai M, Hassan S, Jegadeesan R, Patel HK, Duvvuri A. Should screening for colorectal cancer start at age 45? Gastroenterology 2019;156 Suppl 1:S-949.
- Bilal M, Singh S, Le T-T, Al-Saadi Y, Guturu P. Select group of patients might benefit from early colonoscopic screening for colorectal cancer. Surg Endosc 2020;34:4463-71.
Correspondence: Andres Wonaga
Email: awonaga@yahoo.com.ar
Acta Gastroenterol Latinoam 2023;53(1):59-67
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE