Francisco Schlottmann, Emmanuel E. Sadava, Romina Reino, Martín Galvarini, Rudolf Buxhoeveden
Department of General Surgery, Hospital Aleman of Buenos Aires. Ciudad Autónoma de Buenos Aires, Argentina.
Acta Gastroenterol Latinoam 2017;47(2):117-121
Recibido: 21/06/2016 / Aprobado: 06/03/2017 / Publicado en www.actagastro.org el 03/07/2017
Summary
The role of esophagogastroduodenoscopy (EGD) before bariatric surgery is still under debate. However, because laparoscopic sleeve gastrectomy (LSG) could negatively impact on gastroesophageal reflux disease (GERD), endoscopic findings could change the strategy to a laparoscopic Roux -end- Y gastric bypass (LRYGB). The aim of this study was to evaluate the utility of the EGD before bariatric surgery. A retrospective study based on a prospectively loaded database was performed. A consecutive series of bariatric surgery candidates with preoperative EGD were included. The sample was divided into two groups according to the presence or absence of GERD like symptoms before surgery. G1: patients without symptoms and G2: patients with symptoms. Abnormal EGD findings were defined as presence of hiatal hernia, esophagitis and / or Barrett’s esophagus. A total of 193 patients were included; 123 (63.7 %) patients were female. Median age was 46 (18-71) years. Mean preoperative BMI was 44.5 kg/ m2 (34-96). Distribution among groups was as follows: G1, 136 patients (70 %), and G2, 57 patients (30%). Abnormal EGD findings consistent with GERD were found in 40 patients (29.4 %) in G1 and in 30 patients (52.6%) in G2. LRYGB was performed in 90% of patients with abnormal EGD in G1 and in 80% of patients in G2 patients with abnormal EGD. In conclusion, EGD should be requested as a routine preoperative study in patients undergoing bariatric surgery because its findings could change the surgical strategy.
Key words. Preoperative endoscopy, bariatric surgery, gastroesophageal reflux disease.
La endoscopía preoperatoria en pacientes bariátricos podría cambiar la estrategia quirúrgica
Resumen
La utilidad de la videoendoscopía digestiva alta (VEDA) previa a la cirugía bariátrica es dudosa. Sin embargo, debido a que la realización de una manga gástrica laparoscópica puede impactar negativamente en la enfermedad por reflujo gastroesofágico (ERGE), los hallazgos endoscópicos relacionados a dicha patología podrían cambiar la estrategia quirúrgica hacia un bypass gástrico laparoscópico. El objetivo de este trabajo fue evaluar la utilidad de la VEDA previa a la cirugía bariátrica. Se realizó un análisis retrospectivo sobre una base de datos cargada de manera prospectiva. Se incluyó una serie consecutiva de pacientes candidatos a cirugía bariátrica con realización de VEDA preoperatoria. Se dividió la muestra en dos grupos de acuerdo a la presencia o no de síntomas relacionados a ERGE. G1: pacientes sin síntomas y G2: pacientes con síntomas. Se definió VEDA patológica la presencia de hernia hiatal, esofagitis y/o esófago de Barrett. Se incluyeron 193 pacientes; sexo femenino 123 (63,7%). La edad mediana fue de 46 años (rango: 18-71). La media de índice de masa corporal (IMC) fue de 44,5 kg/m2 (rango: 34-96). Pertenecieron a G1, 136 pacientes (70%) y a G2, 57 (30%). Se evidenció VEDA patológica en 40 pacientes (29,4%) de G1 y en 30 (52,6%) de G2. Se realizó bypass gástrico laparoscópico en el 90 % de los pacientes con VEDA patológica de G1 y en el 80% de los pacientes con alteraciones en la VEDA de G2. En conclusión, la VEDA debería solicitarse como estudio de rutina preoperatorio en pacientes que se someterán a cirugía bariátrica debido a que sus hallazgos podrían cambiar la estrategia quirúrgica.
Palabras claves. Endoscopía, cirugía bariátrica, reflujo gastroesofágico.
Abbreviations
EGD: esophagogastroduodenoscopy.
LSG: laparoscopic sleeve gastrectomy.
GERD: gastroesophageal reflux disease.
LRYGB: laparoscopic Roux -en- Y gastric bypass.
ASMBS: The American Society for Metabolic and Bariatric Surgery.
BMI: body mass index.
EAES: The European Association for Endoscopic Surgery.
ASGE: The American Society for Gastrointestinal Endoscopy.
Obesity is becoming a worldwide increasing concern, and is believed to be the second most preventable cause of mortality in the United States.1 Obese individuals are at increased risk for many health problems including cancer, heart disease, stroke, type 2 diabetes mellitus, hypertension, gastroesophageal reflux disease (GERD) among others.2 Since non-surgical treatment for weight loss in the morbidly obese patients is rarely successful in the long term, bariatric surgery has gained acceptance and popularity in the last years.3
While esophagogastroduodenoscopy (EGD) is mandatory prior to any gastric surgery, the role of EGD before bariatric surgery is still under debate. The American Society for Metabolic and Bariatric Surgery (ASMBS) recommends (with low level of evidence, grade D) to perform preoperative endoscopy only in the presence of clinically significant gastrointestinal symptoms.4 Nevertheless, a lack of correlations between obese patients´ symptoms and endoscopic findings has been documented by many studies.5-8
Laparoscopic sleeve gastrectomy (LSG) is becoming increasingly popular due to its good results and low morbidity.9 Despite its positive effect on weight loss and improvement of comorbidities, several reports have described its negative impact on GERD.10-12 However, endoscopic findings related to GERD could change the strategy to a laparoscopic Roux-en-Y gastric bypass (LRYGB), which is considered the gold standard treatment for the obese patient with GERD.13
The aim of this study was to evaluate the utility of routinely performing EGD prior to bariatric surgery.
Material and methods
An electronic prospectively loaded database of patients who were scheduled for bariatric surgery (LSG or LRYGB) was retrospectively reviewed. All patients who underwent preoperative EGD were included.
Inclusion criteria for bariatric surgery included body mass index (BMI) ≥ 40 or BMI ≥ 35 with obesity-related comorbidities. Preoperative evaluation by a multidisciplinary team consisted of pulmonary, cardiac, psychological and nutritional evaluation. Screening EGD was performed by the bariatric surgeon (RVB) at our institution in an outpatient facility.
Prior to surgery, each patient was interrogated to rule out the presence/absence of GERD like symptoms. The following symptoms were investigated: heartburn, regurgitation, chest pain, dysphagia, chronic cough, and hoarseness. Symptomatic patients were considered those presenting one or more symptoms listed above at least once a week.
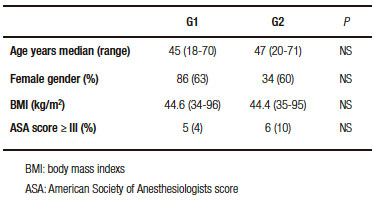
The sample was divided into two groups according to the presence or absence of GERD symptoms before surgery. Group 1 (G1): patients without symptoms and Group 2 (G2): patients with symptoms. Demographics, preoperative BMI, ASA (American Society of Anesthesiologists) score and EGD findings were compared between the two groups.
Abnormal EGD findings related to GERD were defined as follows:
- Hiatal hernia: proximal dislocation of the gastroesophageal junction of ≥ 2 cm above the diaphragmatic indentation.
- Esophagitis: classified following the Los Angeles classification.14
- Barrett’s esophagus: replacement of the stratified squamous epithelium lining of the esophagus by simple columnar epithelium with goblet cells.
All data were analyzed using SPSS v 22.0 (SPSS Inc., Chicago Illinois, U.S.). Chi square/Fisher´s exact test were used for the categorical variables and the student T test for the continue variables. p-values < 0.05 were considered significant.
Results
A total of 193 patients were included. The majority of patients were women (63.7%) and the median age of the entire cohort was 46 (range: 18-71) years. Mean preoperative BMI was 44.5 kg/m2 (range: 34-96). Distribution among groups was as follows: G1: 136 patients (70%) and G2: 57 patients (30%).
No statistical differences in age, gender, BMI or ASA score were found between the two groups (Table 1). Abnormal EGD findings were observed in 40 patients (29.4%) from G1: 22 with hiatal hernia, 15 with esophagitis and 3 with hiatal hernia and esophagitis. Pathological findings were demonstrated in 30 patients (52.6%) from G2: 13 with hiatal hernia, 6 with esophagitis, 8 with hiatal hernia and esophagitis and 3 with Barrett´s esophagus (Table 2).
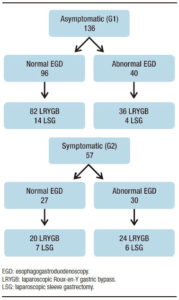
Among asymptomatic patients with abnormal EGD, LRYGB was performed in 36/40 (90%) and LSG in 4/40 (10%). Symptomatic patients with abnormal EGD underwent a LRYGB in 24/30 (80%) cases and LSG in 6/30 (20%). All of the patients with GERD related EGD findings, either asymptomatic or symptomatic, who underwent LSG were scheduled to that type of surgery because of patient´s preference. Surgeries performed in the entire cohort are described in Figure 1.
Table 1. Patient characteristics.
Table 2. Esophagogastroduodenoscopy (EGD) findings.
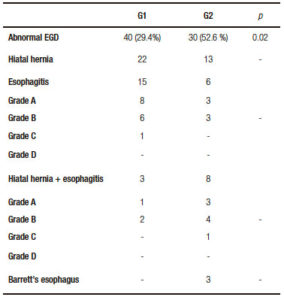
Figure 1. Surgeries performed in the entire cohort.
Discussion
Obesity is an epidemic disease that has suffered an important increacement around the world over the last decade. It is estimated that one third of the US adult population is currently affected.15 Bariatric surgeries has shown to be effective in reducing comorbidities related to obesity as well as overall mortality. Nowadays, LRYGB, laparoscopic adjustable gastric band and LSG are the most common bariatric procedures performed. The latter one has gained worldwide acceptance due to its potential advantages as a simple surgical technique without digestive anastomosis. It is worth of mention, however, that up to 50% of morbidly obese patients present GERD and several publications call into question the benefits of LSG on these patients since it may exacerbate GERD.10-12 Thus, endoscopic findings could be the cornerstone to switch the procedure from LSG to LRYGB which is the gold standard treatment for obese patients with GERD. We evaluated a cohort of patients who underwent EGD during their preoperative workup for bariatric surgery. Abnormal findings such as hiatal hernia, esophagitis and/ or Barrett´s esophagus were diagnosed in one third of asymptomatic patients and over 50% of patients who had symptoms.
Several studies have attempted to address whether LSG exerts a positive or negative influence on GERD, but conflicting results were found. Sheppard and colleagues10 observed that patients with LSG were more likely to develop de novo GERD requiring PPI´s treatment, showing the negative impact of LSG on GERD. Similar results were found by Himpens et al16 with up to 21% of de novo GERD after LSG. Moreover, Gorodner et al11 reported that, 36% of patients in their series developed de novo GERD, and in 21% of patients GERD got worse after LSG. On the other hand, Daes et al17 in a prospective evaluation showed a 94% resolution of GERD-like symptoms, pointing out the need of avoiding relative narrowing at the junction between the vertical and horizontal parts of the stomach. These results suggested that, surgical repair, weight loss and GERD have a complex interaction; thus, understanding their relationship might lead to an improvement of outcomes.
An interesting observation is that most authors usually base their diagnosis of GERD on merely symptoms. It is well known, that symptoms can be misleading at the time of identifying patients with GERD.18 The European Association for Endoscopic Surgery (EAES) guidelines states that, endoscopy, or upper-GI series, are advisable for all bariatric procedures and strongly recommended for LRYGB.19 The American Society for Gastrointestinal Endoscopy (ASGE) recommends endoscopy only in symptomatic patients undergoing bariatric procedures.20 Thus, the role of routine endoscopy before bariatric surgery in asymptomatic patients is unclear. Several authors do not agree with screening endoscopy as it is associated with significantly increase of costs and invasiveness. They also claim that, there is an increase amount of secondary unnecessary workup prompted by irrelevant findings. Schigt et al21 stated that, standard preoperative assessment by EGD in bariatric patients is not indicated because the number needed to screen to find clinically significant abnormalities is high. Gómez et al22 identified age > 55 years and the presence of GERD as risks factors for abnormal findings on screening endoscopy. However, they concluded that although abnormalities on preoperative EGD were frequent, they rarely had changed the surgical management based on those findings. Still, they failed to identify those patients who would not need screening endoscopy before bariatric surgery. Several authors have demonstrated the weak correlation between patients´ symptoms and endoscopic findings; base on this, it has been suggested that routine preoperative endoscopy might be useful in detecting both lesion and inflammation.5, 8, 23 On the other hand, there is evidence that supports changing the bariatric procedure if specific pathologies as hiatal hernia or Barrett´s esophagus are found in the preoperative evaluation.24
In our series, up to 29.4 % of asymptomatic patients showed abnormal findings on endoscopy. These findings highlight the importance of the preoperative EGD as a tool for the surgeon, who might change his/her surgical indication based on its results. We would also like to put emphasis on the advantages of the surgeon performing the EGD him/herself. The surgeon who will operate on that specific patient, and who knows exactly the case, will be able to choose the most adequate procedure for each patient based on his/her own observation. We believe that many asymptomatic patients without preoperative EGD would have been scheduled for LSG, carrying the risk of exacerbating a subclinical GERD or even developing de novo GERD. Some authors suggested that obese patients with documented hiatal hernia during the preoperative evaluation, still refusing a LRYGB, a LSG with hiatal hernia repair could be an acceptable option. Soricelli et al25 reported significant improvement of GERD symptoms after LSG with concomitant hiatal hernia repair, without de novo GERD development.
Limitations of this study include its retrospective design which is the main limiting factor. A relatively small sample was analyzed and the costs of preoperative endoscopy as well as morbidity associated to the procedure were not evaluated. Despite of these limitations, we were able to demonstrate the poor correlation between the presence of GERD-like symptoms and EGD findings and that is the reason why we believe that this study is a relevant contribution in the field of bariatric surgery.
In conclusion, we believe that endoscopy should be performed routinely during the preoperative workup in patients undergoing bariatric surgery. In our minds, there is no question that endoscopic findings could change the surgical strategy. Larger studies are needed to achieve more definitive conclusions.
Financial sustainability. No financial support was provided for this study.
Disclosures. Authors have no conflicts of interest or financial ties to disclose. Drs Schlottmann, Sadava, Reino, Galvarini and Buxhoeveden present no conflict of interest.
References
- Hill JO, Wyat HR, Reed GW, Peters JC. Obesity and the environment, where do we go from here? Science 2003; 299: 853-5.
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and metaanalysis. BMC Public Health 2009; 9: 88.
- American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers 2011 – 2014. Available at https:// asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed 15 December 2015.
- American Society for Metabolic and Bariatric Surgery. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Available at https://asmbs.org/resources/clinical-practice-guidelines-for-the-perioperative-nutritional-metabolic-and-nonsurgical-support-of-the-bariatric-surgery-patient. Accessed 15 December 2015.
- Sharaf RN, Weinshel EH, Bini EJ, Rosenberg J, Sherman A, Ren CJ. Endoscopy plays an important preoperative role in bariatric surgery. Obes Surg 2004; 14: 1367-1372.
- Verset D, Houben J, Gay F, Elcheroth J, Bourgeois V, VanGossum A. The place of upper gastrointestinal tract endoscopy before and after vertical banded gastroplasty for morbid obesitiy. Dig Dis Sci 1997; 42: 2333-2337.
- Coté-Daigneault J, Leclerc P, Joubert J, Bouin M. High prevalence of esophageal dysmotility in asymptomatic obese patients. Can J Gastroenterol Hepatol 2014; 28: 311-314.
- Küper MA, Kratt T, Kramer KM, Zdichavsky M, Schneider JH, Glatzle J, Stüker D, Königsrainer A, Brücher BL. Effort, safety, and findings of routine preoperative endoscopic evaluation of morbidly obese patients undergoing bariatric surgery. Surg Endosc 2010; 24: 1996-2001.
- ASMBS clinical issues committee. Updated statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis 2012; 8: 21-26.
- Sheppard CE, Sadowski DC, de Gara CJ, Karmali S, Birch DW. Rates of reflux before and after laparoscopic sleeve gastrectomy for severe obesity. Obes Surg 2015; 25: 763-768.
- Gorodner V, Buxhoeveden R, Clemente G, Solé L, Caro L, Grigaites A. Does laparoscopic sleeve gastrectomy have any influence on gastroesophageal reflux disease? Preliminary results. Surg Endosc 2015; 29: 1760-1768.
- Burgerhart JS, Schotborgh CA, Schoon EJ, Smulders JF, van de Meeberg PC, Siersema PD, Smout AJ. Effect of sleeve gastrectomy on gastroesophageal reflux. Obes Surg 2014; 24: 1436- 1441.
- Madalosso CA, Gurski RR, Callegari-Jacques SM, Navarini D, Mazzini G, Pereira Mola S. The impact of gastric bypass on gastroesophageal reflux disease in morbidly obese patients. Ann Surg 2016; 263: 110-116.
- Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of esophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999; 45: 172-180.
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006; 295: 1549-1555.
- Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg 2010; 252: 319- 324.
- Daes J, Jimenez ME, Said N, Dennis R. Improvement of gastroesophageal reflux symptoms after standardized laparoscopic sleeve gastrectomy. Obes Surg 2014; 24: 536-540.
- Galvani C, Fisichella M, Gorodner MV, Perretta S, Patti MG. Symptoms are poor indicators of reflux status after fundoplication for gastroesophageal reflux disease. Arch Surg 2003; 138: 514-519.
- Sauerland S, Angrisani L, Belachew M, Chevallier JM, Favretti F, Finer N, Fingerhut A, Garcia Caballero M, Guisado Macias JA, Mittermair R, Morino M, Msika S, Rubino F, Tacchino R, Weiner R, Neugebauer EA; European Association for Endoscopic Surgery. Obesity surgery: evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc 2005; 19: 200-221.
- ASGE standards of practice committee, Anderson MA, Gan SI, Fanelli RD, Baron TH, Banerjee S, Cash BD, Dominitz JA, Harrison ME, Ikenberry SO, Jagannath SB, Lichtenstein DR, Shen B, Lee KK, Van Guilder T, Stewart LE. Role of endoscopy in the bariatric surgery patient. Gastrointest Endosc 2008; 68: 1-10.
- Schigt A, Coblijn U, Lagarde S, Kuiken S, Scholten P, van Wagensveld B. Is esophagogastroduodenoscopy before Roux-en-Y gastric bypass or sleeve gastrectomy mandatory? Surg Obes Relat Dis 2014; 10: 411-418.
- Gómez V, Bhalla R, Heckman MG, Kroner Florit PT, Diehl NN, Rawal B, Lynch SA, Loeb DS. Routine screening endoscopy before bariatric surgery: Is it necessary? Bariatr Surg Pract Patient Care 2014; 9: 143-149.
- Csendes A, Burgos AM, Smok G, Beltran M. Endoscopic and histologic findings of the foregut in 426 patients with morbid obesity. Obes Surg 2007; 17: 28-34.
- De Palma GD, Forestieri P. Role of endoscopy in the bariatric surgery of patients. World J Gastroenterol 2014; 20: 7777-7784.
- Soricelli E, Casella G, Rizzello M, Cali B, Alessandri G, Basso N. Initial experience with laparoscopic crucial closure in the management of hiatal hernia in obese patients undergoing sleeve gastrectomy. Obes Surg 2010; 20: 1149-1153.
Correspondencia: Francisco Schlottmann
Departamento de Cirugía General. Hospital Alemán de Buenos Aires, Ciudad Autónoma de Buenos Aires, Argentina.
Av. Pueyrredón 1640 (ATT 1118)
Tel: +54 (011) 4827 7000 – Fax: +54 (011) 4827 7000 (2807)
Correo electronico: fschlottmann@hotmail.com
Acta Gastroenterol Latinoam 2017;47(2):117-121
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE