Cristiane Kibune Nagasako,1 Sônia Letícia Silva Lorena,2 Célia Regina Pavan,1 Michelle Viviane Sá dos Santos Rondon,1 Ciro Garcia Montes,2 Maria Aparecida Mesquita2
1 Gastrocentro, Universidade Estadual de Campinas. Campinas, Brazil.
2 Disciplina de Gastroenterologia, Departamento de Clínica Médica, Universidade Estadual de Campinas. Campinas, Brazil.
Acta Gastroenterol Latinoam 2016;46: 314-321
Recibido: 23/01/2016 / Aprobado: 07/06/2016 / Publicado en www.actagastro.org el 01/01/2017
Summary
Studies on small intestinal transit in irritable bowel syndrome (IBS) are scarce and inconclusive. The contribution of intestinal dysmotility to the development of small bowel bacterial overgrowth (SIBO) in these patients remains to be elucidated. The aims of this study were to assess the orocecal transit time (OCTT) and the frequency of SIBO in IBS patients, the relationship between these two parameters, and their respective associations with clinical features, body mass index (BMI) and psychological distress. Methods. Ninety consecutive IBS patients were studied. All of them answered a structured questionnaire for demographic and clinical data. The presence of anxiety and depression was assessed by the Hospital Anxiety and Depression scale. Hydrogen breath test after lactulose ingestion (LHBT) and glucose hydrogen breath test (GHBT) were used for the assessment of OCTT and SIBO respectively. IBS group were compared to age-matched healthy volunteers, recruited among the hospital staff (n = 25, OCTT and n = 20, GHBT). Results. OCTT in IBS patients (77 ± 36 min) was significantly higher than in controls (64 ± 40 min; p = 0.03). Individual analysis showed prolonged OCTT (> 80 min) in 28.9% of IBS patients, and SIBO in 15.5%. The only symptom significantly associated with SIBO was diarrhea (p = 0.03). In addition there was a significant relationship (p = 0.005) between SIBO and prolonged OCTT. OCTT was not associated (p > 0.05) with intestinal complaints, BMI, anxiety or depression. Conclusions. About one third of IBS patients present prolonged OCTT, indicating delayed small bowel transit. This abnormality appears to be a predisposing condition for the development of SIBO. SIBO is more frequent in IBS patients with diarrhea.
Key words. Irritable bowel syndrome, orocecal transit time, small intestinal bacterial overgrowth.
La prolongación en el tiempo del tránsito oro-cecal está asociado al sobrecrecimiento bacteriano en el síndrome de intestino irritable. Estudio realizado en un centro terciario en Brasil
Resumen
Los estudios del tiempo de tránsito del intestino delgado en el síndrome de intestino irritable (SII) son escasos e inconclusos. La contribuición de la dismotilidad intestinal en la presencia del sobrecrecimento bacteriano intestinal (SBI) en estos pacientes no esta esclarecida. Los objetivos de este estudio fueron evaluar el tiempo del tránsito oro-cecal (TTOC) y la frecuencia del SBI en los pacientes con SII, la relación entre estos dos parámetros, y sus respectiva asociación con las características clínicas, el índice de masa corporal (IMC) y distress psicológico. Métodos. Fueron estudiados 90 pacientes consecutivos con diagnóstico de SII, siguiendo los criterios de Roma III. Todos respondieron un cuestionario para la determinación de los datos demográficos y características clínicas. La presencia de ansiedad y depresión fue evaluada por la escala hospitalaria de ansiedad y depresión. Los test de H2 espirado después de la ingestión de lactulosa (TRHL) y de glucosa (TRHG) fueron utilizados para investigar el TTOC y el SBI, respectivamente. Los pacientes con SII fueron comparados con voluntarios sanos apareados por edad, (y sexo?) reclutados entre el personal del hospital (n = 25, TRHL y n = 20, TRHG). Resultados. El TTOC en los pacientes con SII fue significativamente más prolongado en relación a los controles (77 ± 36 vs 64 ± 40 min, respectivamente; p = 0,03). El análisis individual mostró TTOC prolongado (>80 minutos) en el 28.9% de los pacientes con SII y SBI en 15.5%. El único síntoma asociado con el SBI fue la diarrea (p = 0,03). Además, hubo correlación significativa entre SBI y TTOC prolongado (p = 0,005). El TTOC no está asociado con molestias intestinales, IMC, ansiedad y depresión (p = NS). Conclusión. Aproximadamente un tercio de los pacientes con SII presentan un TTOC prolongado, indicando un tránsito lento del intestino delgado. Esta anormalidad parece predisponer la presencia del SBI. El SBI es más frecuente en pacientes con SII con diarrea.
Palabras claves. Síndrome de intestino irritable, tiempo de tránsito oro-cecal, sobrecrecimiento bacteriano intestinal.
Abbreviations
IBS: irritable bowel syndrome.
GI: gastrointestinal motility.
BMI: body mass index.
SIBO: small bowel bacterial overgrowth.
LHBT: hydrogen breath test after lactulose ingestion.
GHBT: glucose hydrogen breath test.
OCTT: orocecal transit time.
IBS-C: IBS with constipation.
IBS-D: IBS with diarrhea.
IBS-M: mixed IBS.
HAD: Hospital anxiety and depression scale.
PPI: proton pump inhibitor.
Irritable bowel syndrome (IBS) is a functional bowel disorder characterized by recurrent abdominal pain or discomfort associated with altered bowel habit.1 The pathophysiology of this condition involves the interaction of several mechanisms including abnormal gastrointestinal (GI) motility, visceral hypersensitivity, immunologic and genetic factors, psychological disturbances and alterations in the intestinal microbiota.2-4
Abnormal motor patterns have been described in the whole GI tract of IBS patients, but their role in the pathogenesis of this condition remains to be clarified.5 In particular the studies of small bowel motility in IBS have shown inconsistent results. Manometric evaluation showed evidence of small intestinal motility abnormalities in some patients, but the clinical significance of these alterations is not clear.6 In addition, the results of small bowel transit studies have been contradictory. A few reports showed alterations in the intestinal transit related to bowel patterns7, 8 while others9 found no significant change in small bowel motor function in IBS. Moreover, most studies did not take into account the potential influence of factors that might affect intestinal transit, such as body mass index (BMI) and psychological distress.10, 11
On the other hand, numerous reports have described a higher frequency of small intestinal bacterial overgrowth (SIBO) in IBS.12 However, there is a great variation in the rates of SIBO in different studies, depending on the used diagnostic technique. The lactulose hydrogen breath test (LHBT) has been largely employed as a noninvasive method for the diagnosis of SIBO in the initial studies in IBS. However, the results of those studies have been questioned, particularly for the methodological criteria used, and it seems that the frequency of SIBO was over-estimated with this method.13 Subsequent studies have shown that the glucose hydrogen breath test (GHBT) has a higher diagnostic accuracy for SIBO than the LHBT,14, 15 and this method has been increasingly accepted as a useful diagnostic tool for this condition.16
It is well known that several factors may predispose to the development of bacterial overgrowth, including hypo-aclorhydria due to prolonged use of proton pump inhibitors and previous gastrointestinal surgery (for example Billroth type II partial gastrectomy). In addition, alteration of intestinal motility due to several conditions (e.g. Crohn’s disease, diabetes mellitus and scleroderma) can frequently lead to SIBO.17 However, the relationship between intestinal dysmotility and SIBO in IBS remains to be elucidated. The few reports on intestinal manometry in IBS patients with SIBO were inconclusive,18, 19 and there is a lack of studies investigating small bowel transit in these patients.
Therefore, the aims of this study were to assess: 1) whether there are alterations in the orocecal transit time (OCTT) in IBS patients, 2) the frequency of SIBO in these patients using the GHBT, 3) the relationship between SIBO and OCTT, 4) the respective associations of SIBO and OCTT with clinical features, BMI and psychological distress.
Patients
Ninety consecutive patients (females: 75, males: 15; mean age: 49.6 ± 11 years) attending the outpatient gastroenterology clinic of our university hospital with the diagnosis of IBS participated in the study. IBS was diagnosed on the basis of Rome III criteria.1 Exclusion criteria were previous GI tract surgery, diabetes mellitus, associated diseases that might cause intestinal dysmotility, use of medications known to influence GI transit and use of antibiotics during the 2 months preceding the study.
A standardized questionnaire was used to obtain information about demographic and clinical data. According to bowel habits and the Bristol stool form scale,20 IBS patients were divided into: IBS with constipation (IBS-C), IBS with diarrhea (IBS-D) and mixed IBS (IBS-M). BMI was calculated for all patients. According to the World Health Organization classification,21 patients were classified into underweight (BMI < 18,50), overweight (BMI between 25 and 29.9 kg/m2), obese (BMI ≥ 30 kg/m2) and normal range (18.50-24.99 kg/m2).
Controls
The control group for OCTT included 25 healthy volunteers (mean age: 47 ± 13 years; females: 13), and the control group for GHBT consisted of 20 healthy volunteers (44 ± 15 years; females: 11). Both control groups were recruited among the hospital staff and were matched with IBS patients by age. None of the subjects reported gastrointestinal symptoms or any condition described above as exclusion criteria for IBS patients. No subject had constipation or diarrhea, as defined by Rome III criteria.1
Anxiety and depression
The presence of anxiety and depression was assessed by the previously validated Portuguese version of the Hospital Anxiety and Depression (HAD) scale divided into subscales for anxiety and depression.22 The score for each subscale ranges from 0 to 21. Scores higher than 8 in either HAD subscales were considered to indicate anxiety or depression, respectively.
OCTT measured by the hydrogen breath test after lactulose ingestion (LHBT)
The method used for the LHBT was previously reported in our previous study.23 On the day before the test, the subjects were asked to eat a carbohydrate-free diet. After a 12-h overnight fast, they had a mouth washing with 50mL of 1% chlorhexidine solution. Then, two end-expiratory breath air samples were collected with a 10-min interval. Following that, the participants ingested a meal consisting of two scrambled eggs and drank 200 ml of tea containing 10 g of lactulose. Breath air samples were then taken every 10 min up to 240 min. During the test period, they were not allowed to exercise, drink, eat, or smoke. In each collected sample, hydrogen concentration in parts per million (ppm) was measured on a gas chromatograph (model 12 Microlyzer; QuinTron Instrument Co). The mean hydrogen concentration of the two fasting samples was taken as the base line value. OCTT was defined as the time between the lactulose ingestion and a sustained increase in breath hydrogen of at least 10 ppm above the basal values.24
Glucose hydrogen breath test (GHBT) to assess SIBO
The procedure for the GHBT was similar to that described for the LHBT. After collection of the basal end-expiratory breath air samples, patients ingested 50g of glucose dissolved in water, and breath air samples were taken every 10 min for 120 min. The test was considered positive for SIBO if there was an increase over the baseline H2 level >12 ppm.16, 25
Fecal fat
Fecal fat was estimated by microscopic examination of stool specimens stained with Sudan III according to the method of Drummey et al.26 The presence of more than 100 fecal fat droplets per high power field on microscopy was considered abnormal, indicating steatorrhea.
Ethical approval
The study was approved by the institutional ethics committee. Each patient and healthy volunteer gave written informed consent before participation in the study.
Statistical analysis
Comparisons of the results were performed by the Mann-Whitney U test, Student’s t test, ANOVA, chi-square test and Fisher’s exact test as appropriate. Logistic regression was utilized to analyze the parameters associated with OCTT and SIBO. Correlations between OCTT values and HAD scores were assessed using the Spearman correlation coefficient. All statistical analyses were carried out using SPSS version 17.0 for Windows (SPSS, Inc., Chicago, IL). A value of p < 0.05 was considered to be statistically significant.
Results
Clinical features
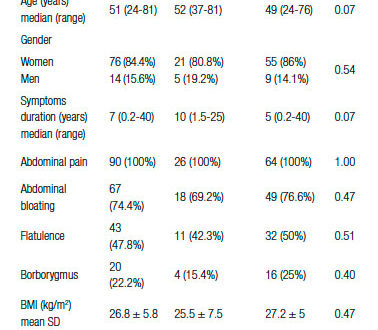
Demographic and clinical data of IBS patients are shown in Table 1. It can be seen that there was a preponderance of women in our series (84.4%). Besides abdominal pain, bloating and flatulence were symptoms frequently reported by IBS patients. According to IBS subtypes, 46 patients (51.1%) were IBS-D, 27 (30%) were IBS-C and 17 (18.9%) were IBS-M.
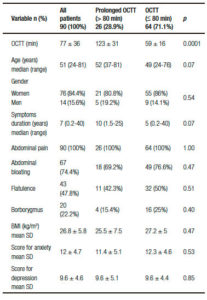
Table 1. Demographic and clinical features of IBS patients. Comparisons between patients with prolonged OCTT (> 80 min) and those with OCTT ≤ 80 min.
BMI
The mean BMI was 26.8 ± 5.8 kg/m2. Twenty-seven (30%) patients were overweight, 24 (26.7%) were obese and 2 (2.2%) were underweight.
Anxiety and depression
Eighty-four patients completed the HAD scale. According to the scale scores, 69 (82.1%) had anxiety and/ or depression. Twenty patients (23.8%) had anxiety, four had depression (4.8%), and 45 (53.6%) had anxiety associated with depression. The mean scores for anxiety and depression are shown in Table 1.
OCTT
Controls
The mean value of OCTT in the control group was 64 ± 40 min. Considering the normal range of the test as the 95% CI (47-80 min), OCTT values > 80 min were regarded as prolonged OCTT. There was no significant difference in OCTT between men and women (p = 0.27), but a significant correlation was found between OCTT values and age (r = 0.65; p = 0.0004).
IBS patients
The mean value of OCTT in IBS patients was 77 ± 36 min. OCTT was significantly correlated with age (r = 0.24; p = 0.02), similarly to the finding in the control group. There was no significant difference in OCTT values between men and women (p > 0.05), or among IBS subgroups (IBS-C: 71 ± 40min; IBS-D: 81 ± 37min and IBS-M: 79 ± 29 min; p = 0.52).
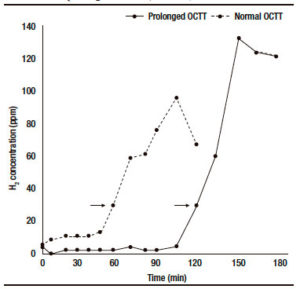
OCTT was significantly higher in IBS patients than in the control group (p = 0.03). Individual analysis showed that 26 patients (28.9%) had prolonged OCTT (> 80 min). Figure 1 shows the results of the LHBT in two patients: one with normal OCTT and the other with prolonged OCTT. The comparisons between IBS patients with prolonged OCTT and those with OCTT (≤ 80 min) are presented in Table 1. There were no significant differences between the two subgroups in the frequency of symptoms of bloating and flatulence, symptoms duration, BMI, and the scores for anxiety or depression. In addition there was no correlation between OCTT values and the scores for anxiety (p = 0.61) or depression (p = 0.66).
Figure 1. Examples of lactulose hydrogen breath test (LHBT) results in two IBS patients: one with normal OCTT (60 min) and the other with prolonged OCTT (120 min).
SIBO
No subject in the control group had a positive GHBT for SIBO.
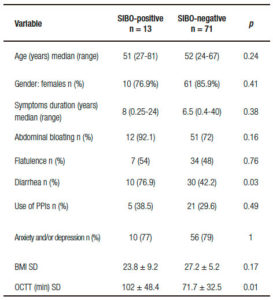
GHBT was performed in 84 IBS patients. The test was positive for SIBO in 13 (15.5%). The comparisons between IBS patients with or without SIBO are presented in Table 2. Ten out of 13 (76.9%) patients with SIBO reported diarrhea (IBS-D: 9; IBS-M: 1), while this alteration was reported by 30 (42.2%) patients without SIBO (p = 0.03). There were no significant differences between SIBO-positive and SIBO-negative patients in age, gender, symptoms of bloating and flatulence, presence of anxiety/depression, use of protom pump inhibitors (PPI) or BMI values. According to BMI, one patient with SIBO was underweight (7.7%), 4 were overweight (30.7%) and two were obese (15.3%).
Table 2. OCTT values and clinical features in IBS patients with and without SIBO (n = 84).
Relationship between OCTT and SIBO
OCTT values were significantly higher in patients with SIBO (102 ± 48.4min) in comparison with those without SIBO (71.7 ± 32.5; p = 0.01). In addition there was a significant relationship (p = 0.005) between SIBO and prolonged OCTT (odds ratio: 5.9; 95% CI: 1.7- 20.1).
Fecal fat
All IBS patients had the examination of stool specimens stained with Sudan III. There was no case of steatorrhea.
Discussion
This prospective study showed prolonged OCTT in 28.9% of IBS patients, indicating delayed intestinal transit in this subgroup. The hydrogen breath test used here for the assessment of OCTT has been widely employed in small bowel transit studies in several conditions25, 27 Studies on small bowel transit in IBS are scarce, and the clinical relevance of the described alterations remains to be elucidated. Earlier studies using the breath hydrogen tests have shown that small bowel transit times were longer in IBS patients with constipation, and shorter in those with diarrhea.7, 28 More recently Agrawal et al29 reported OCTT values greater than the normal reference range in a proportion (17%) of IBS patients with constipation. In the present study no significant association was found between OCTT values and abdominal symptoms. In addition, OCTT did not differ among IBS subtypes. This result is in agreement with the observations of Camilleri et al9 showing that small bowel transit assessed by scintigraphy was similar among IBS subgroups. On the other hand, the authors found a relationship between delayed colonic transit and IBS-C, and accelerated transit with IBS-D. These results suggest that alterations in bowel function in IBS are mainly related to disturbances of colonic transit.
A positive correlation between OCTT values and age was observed in both healthy volunteers and IBS patients of the current study, confirming previous observations that OCTT is longer in healthy elderly.30 This finding highlights the importance of well matched control groups in relation to age in OCTT studies.
The etiology of small intestinal dysmotility in IBS has not been elucidated. Disturbances in intestinal motility could be secondary to external factors, such as the use of medications, or could reflect dysfunction at the level of the gut muscle, or the autonomic or central nervous system.6
In the current study no relationship was found between BMI and OCTT. It should be noted that a large proportion of our IBS patients were overweight or obese, which is consistent with recent data on the Brazilian population.31 There are conflicting reports in the literature regarding the alterations of small bowel transit in obesity. OCTT in overweight/obese subjects has been found to be prolonged, faster or similar to the control group.10, 32 The high prevalence of anxiety and depression in our patients is consistent with other reports in IBS.33 No association was found between anxiety and depression scores and OCTT values, in agreement with the results reported in two recent studies.9, 29 Therefore our results suggest that prolonged OCTT in our IBS patients was not related to BMI, anxiety or depression. Further studies are necessary to investigate other parameters which might be associated with delayed intestinal transit in IBS, including autonomic dysfunction.
Although the current gold standard test for diagnosis of SIBO is the culture of intestinal aspirate, this method is invasive and difficult to be used in clinical practice.34 The non-invasive GHBT used in the current study has been shown to be more specific than the LHBT, and glucose has now become the preferred substrate for breath tests to detect SIBO.35 The 15.5% prevalence of SIBO in our IBS patients is within the 8.5% to 46% rates reported in previous studies using GHBT.12, 36 Furthermore, our result showing a significant relationship between SIBO and diarrhea is in agreement with previous observations in IBS.36, 37 One limitation of the present study is that we did not estimate breath methane. About 14,5% of IBS patients have methane producing gut flora,38 usually associated with constipation and lumpy stools.39, 40 These subjects excrete lower amounts of hydrogen38 and the frequency of SIBO, especially in IBS-C group, could be underestimated.
It is interesting to notice that no patient with SIBO had steatorrhea. This finding is in line with the hypothesis that in IBS patients, SIBO occurs more distally in the ileum, causing an increased fermentation of dietary carbohydrates and consequently leading to osmotic diarrhea, without causing an evident malabsorption syndrome.36, 41
There was no significant difference in BMI values between SIBO-positive and SIBO-negative patients, suggesting that the nutritional consequences of SIBO in IBS are generally mild. However, it should be noted that a complete nutritional analysis depends on several other parameters besides BMI. Therefore additional studies are warranted to obtain a more comprehensive evaluation of possible nutritional deficiencies in these patients.
The present study showed the relationship between prolonged OCTT and SIBO in IBS, suggesting that delayed intestinal transit predisposes the development of SIBO in IBS, as already shown for other conditions. It is well known that delayed intestinal transit prevents intestinal clearance, and this may result in bacterial overgrowth. No other predisposing condition for SIBO was detected in our patients, since none of them had previous abdominal surgery, diabetes mellitus, associated diseases that might cause intestinal dysmotility, or were in use of medications known to influence GI transit. Moreover, PPIs use did not differ between patients with and without SIBO.
Therefore it seems reasonable to suppose that pharmacological agents which accelerates intestinal transit might decrease the incidence of SIBO in IBS patients. Further studies are necessary to test this hypothesis.
However the present study was conducted at a tertiary referral center, and the observed alterations may not apply to IBS patients seen in primary care. In addition, we provided no data regarding the effects of antibiotic treatment on intestinal symptoms, especially diarrhea, in order to confirm the role of SIBO in the generation of symptoms in these patients. Further studies with larger series of patients with IBS and SIBO are warranted to assess whether the treatment with antibiotics results in improvement of abdominal symptoms and diarrhea.
Conclusion
About one third of IBS patients present prolonged OCTT, indicating delayed small bowel transit. This abnormality appears to be a predisposing condition for the development of SIBO. SIBO is more frequent in IBS patients with diarrhea.
Disclosures. All authors declare no conflict of interest.
Agradecimientos. Esther Antonia Sánchez Abrego por el apoyo recibido para esto trabajo.
Sostén financiero. el estudio no posee financiación externa.
Referencias
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006; 130: 1480-1491.
- Lee YJ, Park KS. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol 2014; 20: 2456-2469.
- Mayer EA, Savidge T, Shulman RJ. Brain–Gut Microbiome Interactions and Functional Bowel Disorders. Gastroenterology 2014; 146: 1500-1512.
- DuPont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther 2014; 39: 1033-1042.
- Bellini M, Gambaccini D, Stasi C, Urbano MT, Marchi S, Usai- Satta P. Irritable bowel syndrome: a disease still searching for pathogenesis, diagnosis and therapy. World J Gastroenterol 2014; 20: 8807-8820.
- Quigley EMM. Disturbances in small bowel motility. Best Pract Res Clin Gastroenterol 1999; 13: 385-395.
- Cann P, Read N, Brown C, Hobson N, Holdsworth C. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut 1983; 24: 405-411.
- Sadik R, Björnsson E, Simrén M. The relationship between symptoms, body mass index, gastrointestinal transit and stool frequency in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol 2010; 22: 102-108.
- Camilleri M, Mckinzie S, Busciglio I, Low P, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective Study of Motor, Sensory, Psychologic, and Autonomic Functions in Patients With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol 2008; 6: 772-781.
- Basilisco G, Camboni G, Bozzani A, Vita P, Doldi S, Bianchi PA. Orocecal transit delay in obese patients. Dig Dis Sci 1989; 34: 509-512.
- Gorard D, Gomborone J, Libby G, Farthing M. Intestinal transit in anxiety and depression. Gut 1996; 39: 551-555.
- Ghoshal UC, Srivastava D. Irritable bowel syndrome and small intestinal bacterial overgrowth: Meaningful association or unnecessary hype. World J Gastroenterol 2014; 20: 2482.
- Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000; 95: 3503-3506.
- Rana S V, Sharma S, Kaur J, Sinha SK, Singh K. Comparison of lactulose and glucose breath test for diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Digestion 2012; 85: 243-247.
- Rana S V, Sharma S, Malik A, Kaur J, Prasad KK, Sinha SK, Singh K. Small Intestinal Bacterial Overgrowth and Orocecal Transit Time in Patients of Inflammatory Bowel Disease. Dig Dis Sci 2013; 58: 2594-2598.
- Gasbarrini A, Corazza GR, Gasbarrini G, Montalto M, Di Stefano M, Basilisco G, Parodi A, Usai-Satta P, Vernia P, Anania C, Astegiano M, Barbara G, Benini L, Bonazzi P, Capurso G, Certo M, Colecchia A, Cuoco L, Di Sario A, Festi D, Lauritano C, Miceli E, Nardone G, Perri F, Portincasa P, Risicato R, Sorge M, Tursi A; 1st Rome H2-Breath Testing Consensus Conference Working Group. Methodology and Indications of H 2 -Breath Testing in Gastrointestinal Diseases: the Rome Consensus Conference. Aliment Pharmacol Ther 2009; 29: 1-49.
- Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol 2010; 16: 2978-2990.
- Pimentel M, Soffer EE, Chow EJ, Kong Y, Lin HC. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci 2002; 47: 2639-2643.
- Posserud I, Stotzer P-O, Bjornsson ES, Abrahamsson H, Simren M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007; 1; 56: 802-808.
- Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32: 920–924.
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization Technical Report Series 2000; 8948: 1–253.
- Botega NJ, Bio MR, Zomignani MA, Garcia C, Pereira WA. Transtornos do humor em enfermaria de clínica médica e validação de escala de medida (HAD) de ansiedade e depressã Rev Saude Publica 1995; 29: 355–363.
- Lorena SLS, Souza-Almeida JR, Mesquita MA. Orocecal Transit Time in Patients With Functional Dyspepsia. J Clin Gastroenterol 2002; 35: 21–24.
- Scarpellini E, Abenavoli L, Balsano C, Gabrielli M, Luzza F, Tack J. Breath tests for the assessment of the orocecal transit time. Eur Rev Med Pharmacol Sci 2013; 17: 39-44.
- Ghoshal UC. How to Interpret Hydrogen Breath Tests. J Neurogastroenterol Motil 2011; 17: 312–317.
- Drummey GD, Benson JA, Jones CM. Microscopical examination of the stool for steatorrhea. N Engl J Med 1961 12; 264: 85-87.
- Rana SV, Malik A. Breath tests and irritable bowel syndrome. World J Gastroenterol 2014; 28; 20: 7587–7601.
- Corbett CL, Thomas S, Read NW, Hobson N, Bergman I, Holdsworth CD. Electrochemical detector for breath hydrogen determination: measurement of small bowel transit time in normal subjects and patients with the irritable bowel syndrome. Gut 1981; 22: 836–840.
- Agrawal A, Houghton LA, Reilly B, Morris J, Whorwell PJ. Bloating and distension in irritable bowel syndrome: the role of gastrointestinal transit. Am J Gastroenterol 2009; 104: 1998- 2004.
- Haboubi NY, Hudson P, Rahman Q, Lee GS, Ross A. Small-intestinal transit time in the elderly. Lancet. 1988 23; 1: 933.
- Brasil estabiliza taxas de sobrepeso e obesidade [Portal Brasil web site] [Internet]. April30. 2014 [cited 2014 Nov 7]. Available from: http://www.brasil.gov.br/saude/2014/04/brasil-estabiliza-taxas-de-sobrepeso-e-obesidade
- Xing J, Chen JDZ. Alterations of gastrointestinal motility in obesity. Obes Res 2004; 12: 1723-1732.
- Shah E, Rezaie A, Riddle M, Pimentel M. Psychological disorders in gastrointestinal disease: epiphenomenon, cause or consequence? Ann Gastroenterol Q Publ Hell Soc Gastroenterol 2014; 27: 224-230.
- Rana SV, Bhardwaj SB. Small intestinal bacterial overgrowth. Scand J Gastroenterol 2008 2; 43: 1030–1037.
- Lupascu A, Gabrielli M, Lauritano EC, Scarpellini E, Santoliquido A, Cammarota G, Gasbarrini A. Hydrogen glucose breath test to detect small intestinal bacterial overgrowth: a prevalence case-control study in irritable bowel syndrome. Aliment Pharmacol Ther 2005; 22: 1157–1160.
- Parodi A, Dulbecco P, Savarino E, Giannini EG, Bodini G, Corbo M, Isola L, De Conca S, Marabotto E, Savarino V. Positive glucose breath testing is more prevalent in patients with IBS-like symptoms compared with controls of similar age and gender distribution. J Clin Gastroenterol 2009; 43: 962–966.
- Ghoshal UC, Srivastava D, Ghoshal U, Misra A. Breath tests in the diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome in comparison with quantitative upper gut aspirate culture. Eur J Gastroenterol Hepatol 2014; 26: 753–760.
- Rana S V, Sinha SK, Sharma S, Kaur H, Bhasin DK, Singh K. Effect of predominant methanogenic flora on outcome of lactose hydrogen breath test in controls and irritable bowel syndrome patients of north India. Dig Dis Sci 2009; 54: 1550–1554.
- Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol 2007; 102: 837–841.
- Grover M, Kanazawa M, Palsson OS, Chitkara DK, Gangarosa LM, Drossman DA, Whitehead WE. Small intestinal bacterial overgrowth in irritable bowel syndrome: association with colon motility, bowel symptoms, and psychological distress. Neurogastroenterol Motil 2008; 20: 998–1008.
- Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA 2004; 292: 852-858.
Correspondencia: Cristiane Kibune Nagasako
Gastrocentro – Unicamp
Rua Carlos Chagas 420, Cidade Universitária. Campinas, Brazil
CEP: 13083-878
Tel.: 55-19-35218565 Fax: 55-19-35218566
Correo electrónico: crisknvc@gmail.com
Acta Gastroenterol Latinoam 2016;46(4): 314-321
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE