Marta Juliana Mantilla1 ID· Ferney Africano1 ID· Juan José Chaves2 ID· Isabel Bolivar3 ID
German Tovar1 ID
1 GASTROCAL, Group of Specialists in Digestive Diseases Foscal, Floridablanca, Colombia.
2 Department of Pathology, Fundación Universitaria de Ciencias de la Salud, Hospital San José, Bogotá, Colombia.
3 Patología Bolivar, Foscal Internacional, Floridablanca, Colombia.
Acta Gastroenterol Latinoam 2021;51(4):457-461
Received: 17/09/2021 / Accepted: 03/11/2021 / Published in www.actagastro.org on 13/12/2021 / https://doi.org/10.52787/QCAJ6371
Summary
Background. Paracoccidioidomycosis is an endemic mycosis in subtropical areas of Latin American countries. Digestive tract involvement is unusual, and the development of ileocolonic ulcers and enterocutaneous fistula is not common. Case report. We present the case of a male patient with no significant history, with a diagnosis of intestinal paracoccidioidomycosis of three years of evolution with development of ileocolonic ulcers and enterocutaneous fistula. Conclusions. This case reinforces the importance of suspecting paracoccidioidomycosis in these cases, especially in endemic areas, since its clinical presentation may be similar to other inflammatory intestinal conditions.
Keywords. Paracoccidioidomycosis, endemic mycosis, digestive tract, Colombia.
Paracoccidioidomicosis como causa de úlceras ileocolónicas y fístula enterocutánea. Reporte de un caso
Resumen
Antecedentes. La paracoccidioidomicosis es una micosis endémica en áreas subtropicales de países latinoamericanos. El compromiso del tracto digestivo es inusual y el desarrollo de úlceras ileocolónicas y fístula enterocutánea no es común. Caso clínico. Presentamos el caso de un paciente masculino sin antecedentes de importancia, con diagnóstico de paracoccidioidomicosis intestinal de tres años de evolución y desarrollo de úlceras ileocolónicas y fístula enterocutánea. Conclusiones. Este caso refuerza la importancia de sospechar paracoccidioidomicosis en este tipo de casos, especialmente en áreas endémicas, ya que su presentación clínica puede ser similar a otras condiciones inflamatorias intestinales.
Palabras claves. Paracoccidioidomicosis, micosis endémica, tracto digestivo, Colombia.
Introduction
Paracoccidioidomycosis (PCM) is a systemic mycosis caused by dimorphic fungi of the genus Paracoccidioides. Adolfo Lutz made its first description in Brazil in 1908.1 There are two known species that can cause disease: Paracoccidioides brasiliensis and P. lutzii.2
It is an endemic infection in subtropical areas of Latin American countries, with a high prevalence in Brazil, Colombia, Venezuela, and Ecuador.3 Since its notification is not mandatory in most countries, there are no precise epidemiological data, so prevalence, incidence, and morbidity data are based on reports from epidemiological surveys, case series, and hospitalization records. According to previous studies, the estimated annual incidence in Brazil is between 0.71 and 0.96 per 100,000 people per year.4 In Colombia, the cases detected between 1970 and 1999 allow estimating a prevalence of 32.4 cases per year and an incidence rate between 0.1 and 2.4 cases per 100,000 people per year.5,6
Most patients (>95%) are asymptomatic, and the infection will be detected only by intradermal test. There are two clinical forms of presentation in symptomatic cases: the juvenile (acute/subacute) and the chronic form in the adult. The juvenile form is responsible for 5 to 25% of cases. The clinical manifestations generally include enlarged lymph nodes, hepatosplenomegaly, fever, weight loss and anorexia, which appear in the weeks following the acquisition of infection. Symptoms can also include digestive manifestations, cutaneous and mucosal lesions, osteoarticular involvement and, rarely, pulmonary involvement. The chronic form is the most common (74-76% of cases); its onset is progressive and symptoms can persist for months. In 90% of the cases of chronic PCM, the involvement is pulmonary. In addition, the upper aerodigestive pathway and the skin are also involved.7 Apart from the mouth, other parts of the digestive tract are rarely affected. Case reports predominantly involve the jejunum, ileum, and proximal large intestine.8
We present the case of a male patient with no significant history with a diagnosis of intestinal PCM of three years of evolution with development of ileocolonic ulcers and enterocutaneous fistula.
Case Report
A 39-year-old man from a rural area of Colombia attends a gastroenterology consultation, due to recurrent abdominal pain associated with liquid stools that alternated with episodes of constipation in the last three years. The patient also reported a loss of 30 kg in the same period. In addition, over the previous six months, the pain was predominantly located in the right iliac fossa with subjective mass sensation at that level. The patient did not report episodes of fever, night sweats, lymphadenopathy, cough or other symptoms. His history included chronic antral gastritis with positive H. pylori, for which he underwent eradication therapy without improvement of the symptoms. Among the laboratory tests performed before the consultation in our medical center, only mild anemia was reported. The blood count did not show leukocyte and platelet alterations. HIV, Hepatitis B, and Hepatitis C serologies were negative. A chest x-ray was unremarkable.
During physical examination, palpation of the abdomen revealed a mass of a complex and painful consistency adhered to deep planes in the right iliac fossa, without peritoneal irritation or palpable adenopathies. Based on the clinical data of the history and the physical examination, it was decided to perform a colonoscopy and an abdominal computed axial tomography (CT) scan.
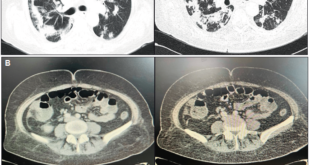
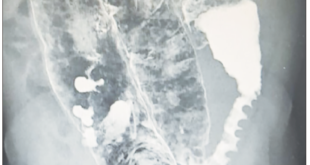
Abdominal CT showed thickening of the walls of the distal ileum at the level of the ileocecal valve, with inflammatory changes in the adjacent fat, and the presence of a fistulous tract with gas that extended to superficial planes (Figure 1). Endoscopic findings revealed erythema and edema with thickening and congestion of the mucosa of the distal ileum, together with ulcerations with a whitish background. Thickening, edema and erythema of the mucosa were also evidenced in the cecum and ileocecal valve. Finally, an ulcer lesion with a whitish background was found in the ascending and transverse colon (Figure 2).
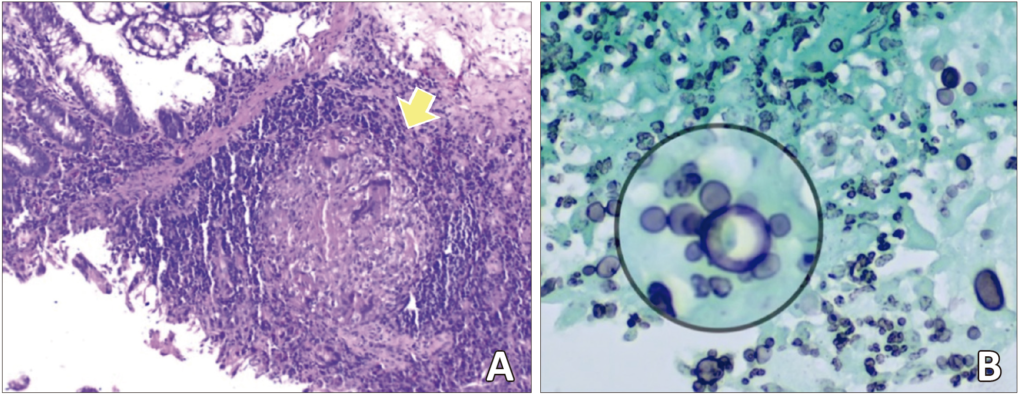
Multiple biopsies were taken, and the histopathological study found chronic and necrotizing granulomatous disease secondary to fungal infection with yeasts morphologically compatible with paracoccidioidomycosis (Figure 3).
Figure 1. Coronal (A) and axial (B) abdominal computed tomography (CT) scan: thickening of the walls of the distal ileum at the level of the ileocecal valve with inflammatory changes in the underlying fat (A). Fistulous tract (yellow arrow) with gas extending to the abdominal wall’s superficial planes (A, B).
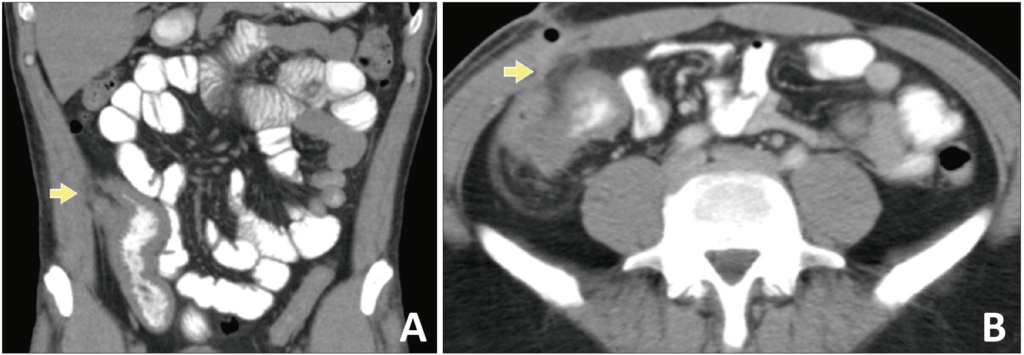
Figure 2. Colonoscopy: ascending colon with the presence of an ulcer with a whitish background of approximately 6 mm (A). Cecum and ileocecal valve with findings suggestive of thickening, congestion, edema, and erythema of the mucosa (B).
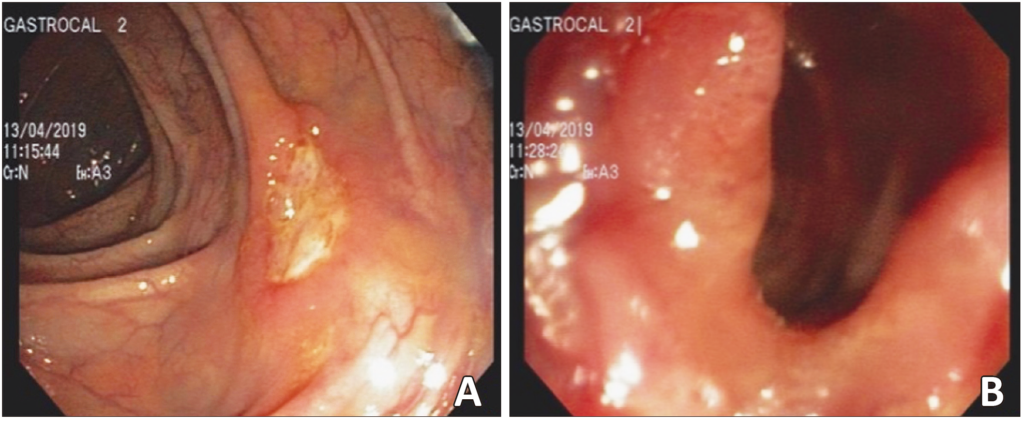
Figure 3. Histopathological study: the presence of granuloma (yellow arrow) with multinucleated giant cells is observed in the wall of the ileocecal region (Hematoxylin & Eosin staining) (A). Multiple, narrow base yeasts with «pilot’s wheel» budding (inside the circle) compatible with Paracoccidioides spp (Grocott methenamine silver staining) (B).
After diagnosis, management with itraconazole 200 mg daily for 12 months with follow-up every three months was indicated. Symptoms improvement were noticeable after the first month of treatment. Regarding the fistula, because its size was small and had no drainage, no procedure or tomographic image control was performed.
Discussion
PCM is a granulomatous disease that can affect any organ of the body, predominantly the lungs, cell-rich organs of the mononuclear phagocytic system, oral/nasal mucosa, and larynx. The digestive tract involvement is compromised in 6.5 to 8.0% of cases.7 Recently, Renz da Cruz et al.8 made a systematic review of intestinal PCM cases, with a total report of 46 cases, being predominant in countries such as Brazil, Peru, Argentina, and Colombia. The average age of the patients was 35.2 years, with a predominance in males (71.1%). It is known that the male patients are more compromised, because the presence of circulating estrogens generates an inhibition of the transformation of conidia to yeast (pathogenic form) and modulates the cellular immune response.2
The main risk factor for acquiring the infection is occupational, related to the management of soils contaminated with the fungus, as it happens working in agriculture, earthworks, soil preparation, gardening and transportation of vegetable products. Unlike other systemic mycoses, PCM is not related to immunosuppressive diseases.7 The acquisition of the disease occurs mainly by inhalation of the conidia. It subsequently invades the lungs in the form of yeast and generates a non-specific inflammatory response. Subsequently, a TH1 response may develop associated with the formation of a granuloma that limits the infection. On the contrary, there is sometimes a TH2 response, associated with a disseminated form with a poor prognosis.9
The most frequent clinical features of the patients with intestinal PCM are abdominal pain (67.3%) and
weight loss (67.3%), followed by diarrhea (65.2%), lymphadenopathy (36.9%), skin manifestations (34.7%),
fever (34.7%), lower gastrointestinal bleeding (30.4%) and oral ulcers (15.2%).8 Constipation is as common as diarrhea, which can last up to 10 days and is usually generated by extrinsic compression or isolated intestinal lesions.3 PCM can affect any segment of the gastrointestinal tract. The ileum and the cecum are the most compromised because they have abundant lymphoid tissue, which is the path of dissemination of the fungus.10
The gold standard for PCM diagnosis is to detect fungal elements suggestive of Paracoccidioides spp in sputum samples or other clinical specimens, such as lymph node aspirates, biopsy specimens, and/or culture with isolation.11 In our case, the diagnosis was made by the histopathological study of biopsy samples from the ileocecal region. In the histopathological examination, oval to round yeasts are observed, which are often provided with multiple peripheral buds (pilot’s wheel configuration). Yeasts often appear in chains and have a single bud. Histological preparations stained with H&E, Grocott methenamine silver, Papanicolaou, or PAS are helpful in revealing fungal elements, especially in granulomatous foci.12
Radiological and endoscopic studies can reveal anatomical or functional abnormalities in a large percentage of patients with PCM of the gastrointestinal tract. In most cases, there is only one segment involvement, being more frequent in the ileum and colon.3 Findings include nodular defects, intestinal loops with irregular contours, ulcerations, stenosis, and external compression of the intestinal segment by lymph nodes.10 The presence of fistulous tracts, as in our case, is unusual. We hypothesize that the formation of the enterocutaneous fistula, in our case, is secondary to the chronicity of the infection.
The most important diagnosis to consider as a differential diagnosis is inflammatory bowel disease, especially Crohn’s disease (CD). CD is an inflammatory disease that can affect any segment of the digestive tract. Symptoms and endoscopic findings (ulcers, stenosis) are similar to PCM findings. For this reason, it is recommended to take intestinal PCM into account in the differentials of patients with symptoms suggestive of CD, especially in endemic countries.8
In terms of management, Paracoccidioides spp is susceptible to most antifungal agents. Despite the significant variability of therapeutic arsenals, the most widely used are itraconazole, cotrimoxazole, and amphotericin B.7 Surgical management is only considered in 15% of cases, especially for the management of complications such as intestinal obstruction.8
Conclusion
We present a case of intestinal PCM, highlighting the importance of taking this differential diagnosis into account, especially in endemic areas. The clinical presentation of intestinal paracoccidioidomycosis may be similar to that other inflammatory conditions. A good clinical history is essential to generate a suspicion of infection by Paracoccidioides spp, which would allow a timely diagnosis and management.
Conflict of Interest. The authors have no conflicts of interest to declare.
Consent for Publication. Written informed consent was obtained from the patient or their parent, guardian, or relative to publish the data and/or clinical images for the benefit of science. A copy of the consent form is available to the editors of this journal.
Intellectual Property. The authors declare that the data and figures that appear in this article are original and were made in their belonging institutions.
Copyright
 © 2021 Acta Gastroenterológica latinoamericana. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license, which allows non-commercial use, distribution, and reproduction, provided the original author and source are acknowledged.
© 2021 Acta Gastroenterológica latinoamericana. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license, which allows non-commercial use, distribution, and reproduction, provided the original author and source are acknowledged.
Cite this article as: Mantilla M J, Africano F, Chaves J J, et al. Paracoccidioidomycosis as a Cause of Ileocolonic Ulcers and Enterocutaneous Fistula. Report of a case. Acta Gastroenterol Latinoam. 2021;51(4):457-1. https://doi.org/10.52787/QCAJ6371
References
- Lutz Adolfo. Uma micose pseudo-coccídica localizada na boca e observada no Brasil: Contribução ao conhecimento das hipho- blastomicoses americanas. Brasil Med. 1908;22:121–4.
- Teixeira MM, Theodoro RC, Nino-Vega G, Bagagli E, Felipe MSS. Paracoccidioides Species Complex: Ecology, Phylogeny, Sexual Reproduction, and Virulence. PLoS Pathogens. 2014 Oct 30;10(10).
- Mendes RP, Cavalcante R de S, Marques SA, Marques MEA, Venturini J, Sylvestre TF, et al. Paracoccidioidomycosis: Current Perspectives from Brazil. The Open Microbiology Journal. 2017 Oct 31;11(1).
- Martinez R. New Trends in Paracoccidioidomycosis Epidemiology. Journal of Fungi. 2017 Jan 3;3(1).
- CALLE D, ROSERO DS, OROZCO LC, CAMARGO D, CASTAÑEDA E, RESTREPO A. Paracoccidioidomycosis in Colombia: an ecological study. Epidemiology and Infection. 2001 Apr 30;126(2).
- Torrado E, Castañeda E, de la Hoz F, Restrepo A. Paracoccidioidomicocis: definición de las áreas endémicas de Colombia. Biomédica. 2000 Dec 1;20(4).
- Shikanai-Yasuda MA, Mendes RP, Colombo AL, Queiroz-Telles F de, Kono ASG, Paniago AMM, et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Revista da Sociedade Brasileira de Medicina Tropical. 2017 Jul 12;50(5).
- da Cruz ER, Forno AD, Pacheco SA, Bigarella LG, Ballotin VR, Salgado K, et al. Intestinal Paracoccidioidomycosis: Case report and systematic review. The Brazilian Journal of Infectious Diseases. 2021 Aug;
- MAMONI R, BLOTTA M. Kinetics of cytokines and chemokines gene expression distinguishes infection from disease. Cytokine. 2005 Oct 7;32(1).
- Goldani LZ. Gastrointestinal Paracoccidioidomycosis. Journal of Clinical Gastroenterology. 2011 Feb;45(2).
- Bernardes Filho F, Sgarbi I, Flávia da Silva Domingos S, Sampaio RCR, Queiroz RM, Fonseca SNS, et al. Acute paracoccidioidomycosis with duodenal and cutaneous involvement and obstructive jaundice. Medical Mycology Case Reports. 2018 Jun;20.
- Restrepo A, Gómez BL, Tobón A. Paracoccidioidomycosis: Latin America’s Own Fungal Disorder. Current Fungal Infection Reports. 2012 Dec 13;6(4).
Correspondence: Marta Juliana Mantilla
Email: mjmantillar9007@gmail.com
Acta Gastroenterol Latinoam 2021;51(4):457-461
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE