Osvaldo M Tiscornia,1, 2 Simmy Bank,3 Fabiana N López Mingorance,1, 2 María B Di Carlo,2 Graciela Otero,1 Selene Rodríguez,1 Patricia G Tiscornia-Wasserman,1, 4 Gustavo A Negri1, 2
1 Programa de Estudios Pancreáticos, Hospital de Clínicas, UBA. Ciudad Autónoma de Buenos Aires, Argentina.
2 Departamento de Bioquímica Clínica, Hospital de Clínicas, FFyB, INFIBIOC, UBA. Ciudad Autónoma de Buenos Aires, Argentina.
3 Deparment of Gastroenterology of Long Island Jewish Medical Center (LIJ). USA.
4 Deparment of Cytopathology, Columbia University. USA.
Acta Gastroenterol Latinoam 2016;46: 291-299
Recibido: 05/01/2016 / Aprobado: 06/06/2016 / Publicado en www.actagastro.org el 01/01/2017
Summary
The aim of the present undertaken was to analyze, by means of the “pancreatogram”, the changes induced by estradiol in a group of non-obese healthy women (n = 15) in the menopause stage (mean age 57) in comparison with a control group (n = 18) of all ages (mean 46). This exam allows an encompassing view of both the exocrine and endocrine pancreas and of their interactions.The main evaluation was done with the 2hs cumulative values of glycemia, insulin, amylase, isoamylase, lipase and calcium. The menopause group was reevaluated after one month of estradiol treatment (estrogen stage) at the dose of 0,625 mg/day. Unexpectedly 75% of the menopause women revealed an impaired glucose tolerance test, this reflected by the glycemia/insulin index figures. The estrogen stage and the one performed following a free hormonal treatment period (post-treatment stage), disclosed a progressive significant decline of the glycemia values associated to unmodified insulin ones. This finding reflects an enhanced sensitivity response to the endocrine hormone. Coupled to the latter are associated the isolated respective values on the lipasemia fall and of calcemia rising. Coherent with these results are those observed with the index figures of lipase/ insulin and lipase/calcium. Undoubtedly estradiol enhances both insulin and calcium secretion. Suggestively, these effects were more marked following a short arrest period of estradiol administration. This fact seemingly allowing to infer that in certain circumstances it would be advisable to choose an intermittent therapeutic option.
Key words. Menopause, estradiol, oral glucose tolerance test.
Páncreas exócrino-endócrino. Menopausia y tratamiento con estradiol. Análisis mediante el “Pancreatograma”
Resumen
El objetivo del presente emprendimiento fue el de analizar, por medio del “Pancreatograma”, los cambios inducidos por el estradiol en un grupo de mujeres no-obesas, sanas (n = 15) en estadío menopáusico (edad promedio 46 años). El aquí propuesto examen faculta el lograr una visión global abarcativa tanto de los componentes exocrino y endocrino del páncreas y el de la interacción entre ambas. La valoración se centró en los valores acumulativos de 2hs de todos los parámetros: glucosa, insulina, amilasa, isoamilasa, lipasa y calcio (estadío pre-tratamiento). El grupo menopáusico fue reevaluado luego de un mes de tratamiento con estradiol en dosis de 0,625 mg/día (estadío estrogénico). Inesperadamente el 75% de las mujeres menopáusicas revelaron una respuesta intolerante de la glucosa, ello bien reflejado por el índice glucosa/insulina. Este estadío, y el desarrollado siguiendo a un período libre de terapia hormonal (estadío post-tratamiento) pusieron de manifiesto una progresiva significativa declinación de los valores glucémicos asociados a niveles invariables de la insulina, ello indicando una incrementada sensibilidad de respuesta de la hormona endocrina. Esto acoplado a una depresión de la lipasemia y a una elevación de la calcemia. Coherente con estos resultados son los observados en los índices lipasa/insulina y lipasa/calcio. Indudablemente el estradiol estimula los valores secretorios de la insulina y del calcio. Fue muy sugestivo que estos efectos fueran más ostensibles siguiendo a un corto periodo de arresto de la terapéutica hormonal. Esto permite inferir que, en ciertas circunstancias, sería aconsejable adoptar un tratamiento intermitente de la hormona femenina.
Palabras claves. Menopausia, estradiol, prueba de tolerancia oral a la glucosa.
Abbreviations
CCK: cholecystokinin.
SHBG: serum-hormone-binding-globulin.
NIDDM: non-insulin-dependent diabetes mellitus.
BMI: body-mass index.
CV: cumulative Value.
Pr. Estr. St.: Pre Estradiol Stage.
Estr. St.: Estradiol Stage.
Post. Estr. St.: Post- Estradiol- Stage.
Lip: lipase.
Ca: calcium.
Ins: insulin.
Gly: glycemia.
Amy: amylase.
HISS: hepatic insulin sensitizing substance.
MISS: meal-induced-insulin-sensitization.
AMISS: absence of meal induced insulin sensitization.
Several decades of involvement in the evaluation of the exocrine pancreas gradually convinced us that it could constitute a practical advance in this field to design a new, easy to perform, non-invasive test able to offer both an encompassing view of the exocrine and endocrine pancreas and of their interactions.1-3
In order to fulfill the above purpose we considered that the classical oral glucosetolerance test might be an appropiate method to start with. It was also our conviction that this exam could be perfected by adding to the classical determinations in blood of glucose and insulin those of total amylase, pancreatic isoamylase, lipase and calcium. We baptized the new procedure as the “Pancreatogram”.
Previous data provided support to this approach. The main ones being those pivoting on insulin, primarily on its trophic and functional influences upon the “pancreon” units that are exerted mainly paracrinically.
Concerning the latter, the changes worthy of emphasis are the divergent influences of stimulation and inhibition, respectively, upon the synthesis and secretion of amylase and lipase.1-5
We have hypothesized that, by evaluating the base line and the post “Pancretogram” parameters values, one might be able to get a trustworthy assessment of both the exocrine and endocrine pancreas and of their interrelationship.
Another background knowledge that was taken into account, especially due to its linking to the present undertaking, is that both the “pancreon” units and the Langerhansislets, as well as the hepatocytes, possess estrogen receptors.6-10 Furthermore, that an intrapancreatic “estrogenic tone” interact with the rest of the neuro-endocrine agents that control the trophism and the secretory capacity of the gland. In that sense, a series of findings of our group support this contention.11-13 One of them is observed analyzing the secretin-induced exocrine pancreatic secretion of healthy men and women above and below the 45 year old age. This study put in evidence that women over this time limit secreted significantly less water and bicarbonate than men. Another report, complementary of the preceding one,12 showed that the above differences could be erased by the administration of estradiol for a month.
In the same line of thought, we also reported, in male rats, that a two-week estradiol treatment elicited a significant potentiation of all basal bile-pancreatic secretion parameters.13 This exocrine secretory change associated, histologically, to a hypertrophy of the Langerhans islets´ beta cells and a marked zymogene granules depletion of the acinar pancreocytes.
Out of the above findings we inferred an estradiol-evoked enhancement of the beta cell insulin secretion. We speculated further that the histological and functional changes might result, on one hand, of a direct effect of insulin, and, on the other, of an interrelationship of this hormone with well known neuro-hormonal factors such as acetylcholine and CCK.14
Linked to the above observations is the clinical-functional feature characterized by a marked secretin-evoked exocrine pancreas hydrelatic hypersecretion. We have put this in evidence in a group of diseases in which are associated, in the liver and pancreas, histological changes of ductular hyperplasia. The entities were those of biliary and alcoholic cirrhosis and hemochromatosis.2 But the fact to be emphasized, that keeps relation with the present report, is the finding of a relative hyperestrogenism resulting from an accentuated peripheral conversion of plasma testosterone and androstenedione to estrogen, phenomenon that is potentiated by chronic alcoholism. In that sense it should be pointed out that ethanol is an agent that increases the number of estrogen receptors in both liver and pancreas.8, 14-16
In respect to the modifications of the estrogenic and of the androgenic tone in different physiological conditions, one to be considered is that of the old men who usually show a fourfold higher levels of estradiol than older women. It should be recalled that in men testosterone is secreted almost exclusively by the testis. Other androgens and some estrogens also come from adrenal secretion and/or the peripheral conversion of precursors. Undoubtedly testicular androgen secretion declines with age. Concerning the increase of estradiol plasma concentration in ageing males this is due partly to its reduced metabolic clearance rate and partly to an increase in peripheral aromatization of androgens.17
In the experimental field one cannot but to make reference to the contribution of the Houssay group in an effort to elucidate the involvement of the sex hormones in the physiology of exocrine-endocrine pancreas.
Houssay18 was one that drew attention to the relation of sex hormones to glucose tolerance. Indeed, in his 1951 review he noted that estrogen given to partially pancreatectomized rats reduced the 6-month incidence of diabetes. Subsequently, his group,19-25 making appeal to the 95 % pancreatectomized rat model, showed that the frecuency of diabetes was higher in males than in females. Besides, that in male rats castration reduced the incidence of diabetes, whereas, in females, inducement of diabetes was substantially increased by ovariectomy. Also, that the protective effect of estrogen was associated with hypertrophy and hyperplasia of both the “pancreon” units and of the Langerhan islets. Furthermore, that the implantation of estrogen pellets in the pancreas was associated with islet regeneration, particularly in the area of the implant. All the above findings undoubtedly pointing to a direct influence of estrogen upon the pancreatic gland.
An observation coherent with the preceding facts is the demonstration that the islets cells coming from rats receiving estradiol for 21 days secrete greater amounts of insulin in respect to those coming from control animals.24
In the same line of the above results are those of Basabe et al.25 Indeed, they showed that long-term administration of estradiol (4 μg/day) for 7 months to ovariectomized rats reduced blood sugar levels by increasing blood insulin values.
The reports above commented and the results of this presentation leave no doubt that estradiol plays a significant role in the physiology of both the “pancreon” units and the Langerhan islets.
Before entering into the details of the present undertaking we have considered appropriated to underscore the contrasting evidences, in respect to the preceding ones that result when androgen tone predominates. Thus, when women show a low level of serum-hormone-binding-globulin (SHBG) which in fact reflects an increase of free testosterone, they usually show signs of insulin resistance and with it visceral obesity and tendency to develop NIDDM, hypertension and cardiovascular disease.26-33
A feature to point out is that, in the hyperandrogenic women the insulin sensitivity in the liver is preserved but reduced in the periphery. An interesting fact, that is pertinent to the findings of the present report, is that the above changes are alleviated by the estrogen administration. Besides that, the female sex hormone improves the glucose metabolism through a depression of the hepatic glucose output.26-33
Another dilemma to be resolved is that of the estradiol-induced enhancement of the insulin responses and, by contrast, those of depression associated by a rising of the testosterone level.26-33
Subjects and methods
Fifteen apparently healthy women, all of them, except two, in spontaneous menopause, were selected from the Clinical Hospital Menopause Clinic. The average age of this Menopause Group was 57.46-56 Their mean body-mass index (BMI) was 23. They were non-smokers and non-alcoholics. None was taking medications known to affect lipid metabolism, nor did they receive sex steroids within the previous 3 months. Post-menopausal status was confirmed by measurements of gonadotropin levels (FSH > 50 IU/ml), (mean 79,1 IU/ml). The post-menopausal women had amenorrhea for > 2 year and estradiol level < 20 μg/ml. Each was free of medical illness, including diabetes.
The study protocol conformed to the guidelines of the World Medical Association Declaration of Helsinki, and to those of the Ethics Committee of the Hospital de Clínicas “José de San Martín”.
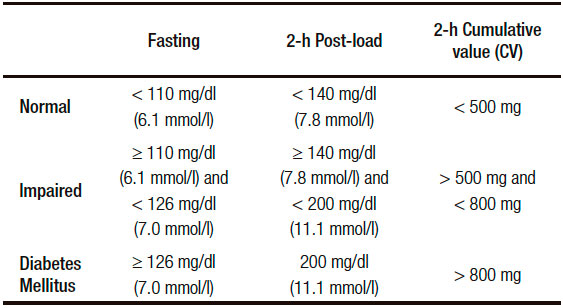
As in this report the analysis of the modifications observed in the “Pancreatogram” play a pivotal role, in the Table 1 is pinpointed, taking essentially into account the blood glucose and insulin values, what was the criteria chosen to include a patient either in a normal, impaired or definite diabetic group.
Table 1. Plasma Glucose Cutpoints Values (fasting and 2-h post Pancreatogram) that define normal glucose metabolism, “impaired” tolerance and diabetes mellitus.
Procedures: Subjects were attended at the “Pancreatic Studies Program” following a 12 hours overnight fast. Height and weight were measured and a general history was obtained including dietary habits, alcohol consumption and family history of diabetes and heart disease.
With the patient semi recumbent a cannula was inserted into an antecubital vein. Blood was taken for basal measurement of plasma glucose,34 insulin by an inmunoenzymatic method, total amylase35 and pancreatic isoamylase previous inhibition of salivary amylase with wheat gluten; lipase by the Lehmann method36 and calcemia by a standard laboratory method.
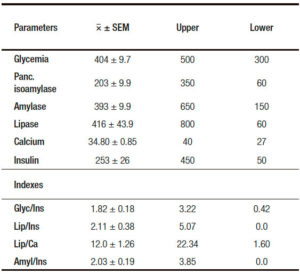
Subsequently, an oral glucolin solution (75 g diluted in 350 ml of tap water) was given by mouth. Blood samples at 30, 60 and 120 minutes were drawn and the same determinations above described were repeated. Other parameters besides those that have been already stated were, i.e, the “Cumulative Value”(CV) (adding fasting value plus those of the post-glucose challenge samples) (Table 2).
In addition, a series of indexes were analyzed, such as: glucose/insulin, amylase/isoamylase, lipase/ insulin and lipase/calcium. The above quotients were calculated with the respective parameters´ values of each sample, fasting and post-glucose testing, including those of the respective “CV”.
In this group of 15 patients we evaluated the effects of one month of estradiol treatment. Also, the eventual modifications of the “pancreatogram”, following a two-month free treatment period. Thus, in order to evaluate the results, a “Pre.Estradiol Stage” (Pr.Estr.St.), an “Estradiol Stage” (Estr. St.) for one month and, following a two-month free treatment period, a “Post- Estradiol- Stage” (Post. Estr. St.) were considered and evaluated comparatively.
The findings of the “Menopause Group” were compared with those of a “Control Group” The latter was constituted by eighteen healthy subjects of all ages (mean 46, range 37-64 yr.) 13 females and 5 men that were subjected to the same “Pancreatogram” above described.
The statistical analysis was performed essentially with the Student “t” test. When appropiate, the non-parametric Mann-Whitney test was also applied. A p value of less than 0.05 was considered as significant.
Table 2. Analysis of the Control Group of the “Pancreatogram” 2-h Cumulative Values CV Indexes, upper and lower. Cutpoint values of each parameter.
Results
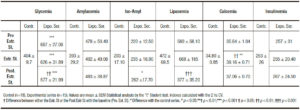
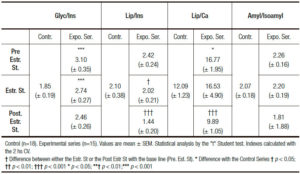
The first finding to emphasize is that 75% of the “Menopause Group” disclosed, unexpectedly, an “impaired glucose tolerance test” (Tables 1-4). The “Menopause Group” showed in the three Stages analyzed significantly higher glycemia values in respect to those of the “Control Group”. Surprisingly the difference was ostensibly less notorious in the Post. Estr. St. This is primarily reflected in the significant fall of the CV of this stage in respect to those of the Pr.Estr. St. (Table 3).
Concerning insulin (Table 3) its value in the three stages of the “Menopause Group” do not differ from those of the Control Group. It is worthy of emphasis that the blood insulin values of the Control Group are similar to those recently reported in Turkey.38
In respect to pancreatic isoamylase (Table 2), this enzyme shows a slight, non significant progressive rise going from the Pr. Estr. St. to the Post.Estr. St. When the analysis is restricted to the evaluation of the CV this is put in evidence in the Post. Estr. St. a significant higher level than in the Control Group.
A feature opposite to the above one is displayed by lipase (Table 3). Indeed this parameter reveals in the CV a significant lower value in the Post.Estr.St. in respect to those of the Pr. Estr. St.
In respect to calcemia (Table 3) estradiol treatment induces a significant rise. This is reflected by the CV level which is significantly higher than those of the Pr.Estr. St. and the Control Group.
Total amylase is in fact the only parameter that does not reveal estradiol-induced changes (Table 3).
The analysis of the different isolated parameters (Table 3) and all their indexes (Table 4) allow confirmation of the above changes associated with estradiol treatment. Thus, the Gly/Ins index shows a progressive value fall in all the different samples during the Estr. St. and the Post. Estr. St. in respect to those of the baseline. This change being more ostensibly clear in the CV results (Table 3). The same phenomenon is displayed by the Lip/Ca index (Table 4). These findings putting in evidence a sex-hormone-induced improved sensitivity of the insulin receptor both in the peripheral tissues (muscle and adipose) and in the exocrine pancreocytes.
Table 3. 2hs cumulative value (CV) of the «AOGTT» parameters in the different stages of the Experimental Series. Analysis of Estradiol treatment effects on Menopausal Women. Each subject analyzed as her own control and comparison with a control group.
Concerning the sex hormone-evoked modifications of the calcemia and of its related indexes, mainly those of Ins/Ca and of Lip/Ca might also be an expression of an enhanced insulin receptor sensitivity and, as a result of that, of an augmented hormone-evoked intracellular calcium release.
In respect to the absence, as expected, of a definitive augmented amylase response following estradiol administration, a finding that would be coherent with an increased sex hormone-induced pancreocytes insulin receptor sensitivity, this is probably well explained by the recent observation that in isolated pancreatic acini estradiol causes a doubling of the intracellular amount of amylase and trypsin but does not affect the secretion of these digestive enzymes into the culture medium.39 Furthermore in other studies in whole rats treated with supplementary dosis of estradiol, their isolated acini showed an elevated amylase content but a reduced secretion rate.39 The latter is in fact in accord with the Blevins et al40, 41 findings in the sense that estradiol treatment induces a decrease of CCK receptors on the cell surface. From the above observations one might infer that the lack of an expected rising of the amylasemia values subsequent to estradiol administration would be the result of an indirect phenomenon, essentially of a decrease of the acinar cell reponsiveness to CCK.
Table 4. Different Indexes of the «Pancreatogram». Analysis of the Estradiol-Induced effects on Menopausal Women (Experimental Series). Comparison with a control group.
Discussion
The first fact to be emphasized resulting of the present undertaking is that the performing of the “Pancreatogram” here proposed discloses, rather unexpectedly, that 75% of a group of healthy non-obese menopause women reveal an impaired glucose tolerance test. This finding associated to normal, except fasting, post-oral glucose-induced blood insulin values. Interestingly, this group during the Estr. St., and surprisingly even more notoriously in the Post. Estr. St., showed an improvement of the “Pancreatogram” parameters. For example, the Gly/ Ins decreased to a level non-significant from that of the control series.
The here observed association of events is well explained according to our view by the report of Spencer et al.42 Indeed, they have pointed out, after analyzing a group of subjects closely similar to the one of our series, that this clinical –endocrinologic condition is associated with a decline of insulin secretion and followed by a progressive increase in insulin resistance and hepatic insulin throughput, i.e., less insulin is taken up by the liver. According to this group, the essential physiological action of the estradiol replacement is that of an increase in both insulin sensitivity and secretion and hepatic insulin uptake.
A feature emphasized by Spencer et al.42 is that oral estradiol therapy in post-menopausal women increases hepatic but not peripheral sensitivity, and that the lowering of glycemia figures results from an estrogen-induced reduction in glucagon secretion and sensitivity. In a few words, that the estrogen effects are less counteracted by glucagon, primarily at the liver. In addition, that the improvement in insulin secretion in response to estrogen replacement is a relatively long-term effect resulting from the islets´beta cells hypertrophy and hyperplasia. In synthesis, that the lack of overall changes in the insulin concentration is consequence of the fact that, its estradiol-induced pancreatic secretion is compensated by a simultaneous enhancement of its hepatic uptake.
According to Spencer et al,42 the estradiol-evoked insulin secretion is primary and the increased insulin uptake by the liver is a secondary effect.
In regard to the above observations, it is appropiate to add those of Anderson et al.26 This group reported an improvement of glucose metabolism and plasma lipids in postmenopausal with NIDDIM after replacement therapy with estradiol. What they emphasize is that, these diabetic women were hyperandrogenic in comparison with non-diabetic women and that after estradiol substitution, SHBG increased 4-fold, whereas free testosterone decreased. According to these authors, hyperandrogenicity, as indicated by low SHBG values, is a powerful independent risk factor for the development of NIDDM, hypertension, and cardiovascular disease. Furthermore, that a low SHBG is associated closely with visceral obesity. Indeed, women with central body fat distribution have low levels of SHBG and increased free testosterone in parallel with insulin resistance. Visceral obesity is a risk factor for cardiovascular disease, stroke and NIDDM. Low SHBG concentration and visceral obesity may have additive effects on insulin resistance. Thus, increased androgenicity in women may cause insulin resistance. On the other hand, there are several pieces of evidence that insulin resistance, or rather hyperinsulinemia, may lead to hyperandrogenism. In effect, it has been shown that hyperinsulinemia increases androgen output from the ovary and may suppress SHBG production in the liver.
That hyperandrogenicity induces insulin resistance and glucose intolerance is in agreement with the results of Anderson et al,26 because elevated SHBG levels induced by estrogen administration, alleviating hyperandrogenicity, were followed, at least partly, by an improved glucose tolerance. It should be pointed out that, insulin sensitivity is preserved in the liver but reduced in the periphery in hyperandrogenic women. In addition to alleviating androgen effects on muscle, estrogen regulates insulin-induced glucose transport via translocation of glucose transporter. In the mechanism for the improvement of glucose homeostasis to the preceding effects should be added those of the estradiol-induced depression of hepatic glucose output.
As a collateral digression it should be mentioned that in a series of papers several interesting properties of estradiol and testosterone has been added. Thus, as the latter causes immunosuppression, estrogen does enhance immune function. The above facts explains the philosophical elaboration that whereas males, on the one hand, are less likely to have autoimmune diseases or reject organ transplant, on the other might be less able to handle a septic challenge after trauma or hemorrhage.
A series of reports, beside those already mentioned in the introduction, provide useful complementary data. One of them is that of Sutter-Dub.43 This author showed, in the isolated perfused pancreas, that estradiol exerts a direct stimulatory effect on the pancreatic islet cells.
Another finding coherent with the above is that reported by Kalkhoff and Kim44 in the sense that estrogens reduce glucagon secretion from islets isolated from pregnant rats. Also that of Faure et al.45, 46 in which the increased glucagon concentration induced by ovariectomy in rats was reversed by the administration of estradiol.
We are convinced that the findings of our report represent, in fact, the first stage of a metabolic disorder that slowly and progressively leads to the insulin resistance syndrome. Although it has been argued that this can be seen in non-obese individuals, it is accepted that insulin resistance most commonly occurs as a result of increasing weight, lack of exercise, aging, diseases or drugs that antagonize the actions of insulin.47
At this stage one cannot but make reference to the original findings of the Canadian school of Lautt.48-51 Indeed, this group, in a series of “in-vivo” and “in-vitro” studies, in the last 15 years have demonstrated that 55% (~66% in humans) of the glucose disposal effect of an I.V. injection of insulin in the fed state, is dependent on the action of a second hormone: “hepatic insulin sensitizing substance” (HISS) which is released from the liver and stimulates glucose uptake in muscle, heart and kidneys. Sensitization of the insulin response by a meal through release of HISS is called meal-induced-insulin-sensitization (MISS). Absence of HISS action results in post-prandial hyperglycemia, hyperinsulinemia, hyperlipidemia, adiposity, increased free radical stress and a cluster of progressive metabolic and cardiovascular dysfunction referred to AMISS (absence of meal induced insulin sensitization). Reduced HISS release accounts for the insulin resistance that occurs with ageing, and is made worse by physical inactivity and diets high in glucose and fat.
The Lautt group proved that HISS acts selectively in skeletal muscle, heart and kidneys, but not liver or adipose tissue, to stimulate glucose uptake.
In order for insulin to cause the release of HISS, two permissive signals are needed. One signal is delivered via hepatic parasympathetic nerves acting on muscarinic receptors and subsequent activation of nitric oxide synthase and elevated cGMP. The other is chemical signal seen as an approximately 40% elevation in hepatic glutathione. Both signals are needed, as either signal, alone is not sufficient to activate the HISS pathway. The feeding signals decrease with the duration of fasting until by 24 hs HISS release is minor or absent. The MISS process has now been demonstrated in mice, rats, guinea pigs, cats, dogs and humans by independent laboratories.
Essentially, then, glucose in the blood causes pulsatile release of insulin, which reaches the liver where reflexively activated parasympathetic nerves and rapidly elevated hepatic glutathione serve as permissive regulators to allow pulses of insulin to stimulate pulsatile releases of HISS. This putative hormone enters the bloodstream and stimulates glucose uptake, primarily into skeletal muscle, doubling the disposal effect of a pulse of insulin. Absence of HISS action results in a major shift in storage of nutrient energy from glycogen in muscle to fat. Absence of HISS action is physiologically and appropriately produced in the fasting state when the hypoglycemic effect of HISS would not be advantageous.
In respect to the behavior of the exocrine pancreas component of the “Pancreatogram”, i.e, pancreatic isoamylase and lipase, only the values of the latter give an indication of an estradiol-induced enhancement of the normal influences exerted by the beta cell insulin upon the neighbouring pancreocytes. Indeed, the fall of isolated lipasemia and of the enzyme-linked indexes (Lip/Ins, Lip/Ca) give testimony of the inhibitory influence normally exerted by insulin upon the synthesis and secretion of this exocrine pancreas enzyme. When commenting the results we have elaborated on the mechanism that could explain the lack of influence of estradiol on the secretion of amylase.39-41 As we have pointed out an interesting possibility is that the mechanism that could explain the lack of an expected estradiol-evoked rising of the amylasemia values is the demostration that this sex hormone inhibits secretion of the enzyme through a decrease of the acinar cell responsiveness to ecbolic agents (acetylcholine, CCK , etc.).30, 41
We are convinced that through the repeated performing of our proposed “Pancreatogram” we will be able, in some clinical circumstances, either to confirm or dismiss that a high lipasemia value, associated to coherent changes of the corresponding indexes (Lip/Ins,Lip/Ca), is a reflection of a definite pancreatic lesion or, by contrast, only a result of a functional derangement of the normal exocrine-endocrine interactions.
To the above inferences we feel that another one must be added. This centered on our conviction that repeating the proposed test through a long-term period will allow to put in evidence, especially in those patients that have followed an adequate treatment, the regeneration capacity of both the exocrine and endocrine components of the pancreatic gland.53-56
Conclusion
The main basis that give support to the approach of the “Pancreatogram” the “insulo-pancreon-axis” interaction, explain that, when confronting the results of a case, in general just a rapid glimpse of its isolated parameters values allow to conclude without hesitation that the test is either normal or allow to infer a certain abnormality.
A feature that deserves to be stressed, well explicited in this case, is the valuable diagnostic support of those physiologic mechanisms of cellular sensitization, primarily involving three indexes, glyc/ins, lip/ins, lip/ca, that are triggered within the “insulo-pancreon” axis.The evolution of the results through time, has shown, in many patients, that the pancreatic gland. Undoubtedly possess an undeniable regeneration capacity.
Referencias
- Tiscornia OM. Concepto de pancreó In: Gastroenterología. Pérez V, De Larrechea I, Arabehety J, Tiscornia OM, eds Bs As: El Ateneo, 1971; 470-484.
- Dreiling D, Tiscornia OM. Test of pancreatic function. In: Sircus W, ed Scientific Foundation of Gastroenterology. London: W. Heinemann Medical Book, 1980; 591-601.
- Tiscornia OM., Lehmann ES de, Hamamura S, Negri G, Otero G, Waisman H.Sistema nervioso autónomo y páncreas. (Análisis de la influencia de diversos tipos de denervación autonómica en los fenómenos de regeneración glandular y en las de las interacciones del eje endocrino-exocrino). A Ge La. 2000; 30: 253-265.
- Duan RD, Wicker C, Erlanson-Albertsson C. Effect of insulin administration on contents, secretion and synthesis of pancreatic lipase and colipase in rats. Pancreas 1991; 6: 595-602.
- Rausch U, Rüdiger K, Vasiloudes P, Kern H, Scheele G. Lipase synthesis in the rat pancreas is regulated by secretin. Pancreas 1986; 6: 552-558.
- Kirdani RY, Sandberg AA, Murphy GP. Estrogen-binding to pancreas. Surgery 1973; 74: 84-90.
- Tesone M, Chasenbalk GD, Ballejos G, Charreau EH. Estrogen receptor in rat pancreatic islets. J of Steroid Biochem. 1979; 11: 1309-1314.
- Kern F Jr, Erfling W, Simon FR. Effect of estrogens on the liver. Gastroenterology 1978; 75: 512-522.
- Boctor AM, Band Ph, Grossman A. Specific binding of 3H-estradiol to the cytosol of rat pancreas and uterus: bound sites in pancreatic extracts do not translocate 3H-estradiol to nuclei suggesting a basic difference in mode of action. J Recep Res 1981- 1982; 2(5-6): 453-463.
- Winborn WB, Sheridan PJ, McGill HC. Estrogen receptors in the islets of Langerhans of baboons. Cell Tissue Res. 1983; 230: 219-223.
- Tiscornia OM. Human exocrine pancreatic responses with different types of secretin. Influence of sex and age. Am J Gastroenterol. 1978; 69: 166-175.
- Tiscornia OM, Cresta MA, Lehmann ES, Belardi G, Dreiling D. Estrogen effects on exocrine pancreatic secretion in menopausal women: A hypothesis for menopausal-induced chronic pancreatitis. Mt Sinai J Med 1986; 53: 356-360.
- Tiscornia OM, Cresta MA, Celener D, Lehmann ES, Tumilasci O, Scacchi P, Dreiling D. Estrogen effects on basal bile-pancreatic secretion and the exocrine-endocrine pancreatic gland in the rat. Mt Sinai J Med 1986; 53: 462-469.
- Saito A, Williams JA, Kanno T. Potentiation of cholecystokinin-induced exocrine secretion by both exogenous and endogenous insulin in isolated and perfused rat pancreata. J Clin Invest. 1980; 65: 772-782.
- Tiscornia OM, Cresta MA, Lehmann ES, Celener D, Dreiling D. Effects of sex and age on pancreatic secretion. Int J Pancreatol 1986; 1: 95-118.
- Renner IG, Rindernecht H, Wisner JR. Pancreatic secretion after secretin and cholecystokinin stimulation in chronic alcoholics with and without cirrhosis. Dig Dis & Sci. 1983; 28: 1089-1093.
- Van Kee PA, Utian WH, Vemeulen A. In: The controversial climateric. Workshop on Menopause. International Congress on the Menopause (3rd: 1981: Ostend, Belgium). Lancaster, England; Boston: MTP Press, 1982; 61-71.
- Houssay BA. Action of sex hormones on experimental diabetes. CIBA Foundation Lecture 1951.Br Med J 1951; 505-510.
- Houssay BA, Biasotti A. The hypophysis, carbohydrate metabolism and diabetes. Endocrinology 1931; 15: 511-523.
- Foglia VG, Schuster N, Rodriguez RR. Sex and diabetes. Endocrinology 1947; 41: 428-434.
- Lewis JT, Foglia VG, Rodriguez RR. The effects of steroids on the incidence of diabetes in rats after subtotal pancreatectomy. Endocrinology 1950; 46: 111-121.
- Borelli MI, Cortizo AM, Gagliardino EE, García ME, Gagliardino JJ. Multiple modulators of the glucose-induced net calcium uptake by isolated islets. Acta Physiol Pharmacol Latinoam. 1984; 34: 1-8.
- Houssay BA, Foglia VG, Rodríguez RR. Production and prevention of some types of experimental diabetes by estrogens or corticosteroids. Acta Endocrinol (Copenh). 1954; 17: 146-164.
- Rodríguez RR.Influence of estrogens and androgens on the production and prevention of diabetes. In: On the Nature and Treatment of Diabetes. Eds, Wrenshall GA, Leibel BS. New York: Excerpta Medica Foundation; 1965; 288-307.
- Basabe JC, Chieri RA, Foglia VG. Action of sex hormones on the insulinemia of castrated female rats. Proc Soc Exp Biol Med. 1969; 130: 1159-1161.
- Andersson B, Mattson L, Hahn L, Marin P, Lapidus L, Holm G, Bengtsson B, Björntorp P. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab.1997; 82: 638-643.
- Barrett-Connor E, Laakso M. Ischemic heart disease risk postmenopausal women. Effects of estrogen use on glucose and insulin levels. Arteriosclerosis 1990; 10: 531-534.
- Mizushima Y, Wang P, Jarrar D, Cioffi WG, Bland KI, Chadry IH. Estradiol administration after trauma hemorrhage improves cardiovascular and hepatocellular functions in male animals. Ann Surg 2000; 232: 673-679.
- Remmers DE, Cioffi WG, Bland KI, Angele MK, Chaudry IH. Testosterone: The crucial hormone responsible for depressing myocardial function in males after trauma-hemorrhage Annals of Surgery 1998; 227: 790-799.
- Jarrar D, Wang P, Knöferl MW, Kuebler JF, Cioffi WG, Bland KI, Chaudry IH. Insight into the mechanism by which estradiol improves organ function after trauma-hemorrhage. Surgery 2000; 188: 246-252.
- Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Arch Surg. 1996; 131: 1186-1192.
- Remmers DE, Wang P, Cioffi WG, Bland KI, Chaudry IH. Testosterone receptor blockade after trauma-hemorrhage improves cardiac and hepatic functions in males. Am J Physiology 1997; 273: H2919-H2925.
- Berry SM, Friend LA, Mc Fadden DW, Brodisch R, Krusch DA, Fink AS. Pancretic denervation does not influence glucose-induced insulin response. Surgery 1994; 116: 67-75.
- Trinder P. Glucemia: Método enzimá (glucose-oxidasa/peroxidasa). Am Clin Biochem.1969; 6: 24-26.
- Junge W. Amylase determination. Clin Biochem. 1989; 22: 109-111.
- Lehmann ES, Tiscornia OM. Valoración de la lipasa en la exploración secretoria pancreá Método de Bang modificado. Pren. Méd. Arg. 1984; 71: 170-171.
- Walczewska A, Yu WH, Kavanth S, Mc Cann SM. Estrogen and leptin have differential effects on FSH and LH release in female rats. Proc Soc Exp Biol Med. 1999; 222: 170-177.
- Sonsuz A, Basaranoglu M, Bilir M, Senturk H, Akin P. Hyperinsulinemia in nondiabetic, both obese and nonobese patients with nonalcoholic hepatitis. Am J Gastroenterol. 2002; 97: 495-496.
- Hilgendorf I, Gellersen O, Emmrich J, Mikkat U, Rohwedel J, Krammer HJ, Müller PK, Kruse C.Estradiol has a direct impact on the exocrine pancreas as demonstrated by enzyme and vigilin expression. Pancreatology 2001; 1: 24-29.
- Blevins GT Jr, Huang HS, Tangoku A, Mckay DW, Rayford PL. Estrogen influence cholecystokinin stimulated pancreatic amylase release and acinar cell membrane cholecystokinin receptors in rat. Life Sci. 1991; 48: 1565-1574.
- Blevins GT Jr, McCullough SS, Wilbert TN, Isom RM, Chowdhury P, Miller ST. Estradiol alters cholecystokinin stimulus-response coupling in rat pancreatic acini. Am J Physiol. 1998; 275: G993-G998.
- Spencer CP, Godsland IF, Cooper AJ, Ross D, Whitehead MI, Stevenson JC. Effects of oral and transdermal 17-b-estradiol with cyclical oral norethindrone acetate on insulin sensitivity, secretion and elimination in postmenopausal women. Metabolism 2000; 49: 742-747.
- Sutter-Dub MT. Preliminary report: Effects of female sex hormones on insulin secretion by the perfused rat pancreas. J Physiol (Paris) 1976; 72: 795-800.
- Kalkhoff RK, Kim HJ.Effects of pregnancy on insulin and glucagon secretion by perfused rat pancreatic islets. Endocrinology.1978; 102: 622-631.
- Faure A, Sutter-Dub MT, Sutter BC. Ovarian-adrenal interactions in regulation of endocrine pancreatic function in the rat. Diabetología 1983; 24: 122-127.
- Faure A, Billaudel B, Sutter BC. Adrenal interference of insulin secretion after 14 days of estradiol treatment in female rats. Diabetología 1984; 26: 76-80.
- Everson SA, Goldberg DE, Helmrich SP, Lakka TA, Lynch JW, Kaplan GA, Salonen JT. Weight gain and the risk of developing insulin resistance syndrome. Diabetes Care 1998; 21: 1637-1643.
- Xie H, Lautt WW. Insulin resistance caused by hepatic cholinergic interruption and reversal by acetylcholine administration. Am J Physiol 1996, 271: E587 – E592.
- Lautt WW. The HISS story overview: a novel hepatic neurohumoral regulation of peripheral insulin sensitivity in health and diabetes. Can J Physiol Pharmacol. 1999; 77: 553-562.
- Patarrão RS, Lautt WW, Afonso RA, Ribeiro RT, Fernandes AB, Boavida JM, Macedo MP. Postprandial but not fasting insulin resistance is an early identifier of dysmetabolism in overweight subjects. Can J Physiol Pharmacol. 2012; 90(7): 923-931.
- Wang HH, Chowdhury KK, Lautt WW. A synergistic, balanced antioxidant cocktail, protects aging rats from insulin resistance and absence of meal-induced insulin sensitization (AMIS) syndrome. Molecules 2015;20(1):669-682.
- Tiscornia OM, Negri GA, Otero G, Lopez Mingorance F, Waisman H, Tiscornia-Wasserman P. Pancreatic polypeptide: a review of its involvement in neuro-endocrine reflexes, islet acinar interactions and ethanol-evoked physiopathologic pancreatic gland changes. Acta Gastroenterol Latinoam. 2015, 45: 155-164.
- Tiscornia OM, Dreiling DA. Does the pancreatic gland regenerate? Gastroenterology 1966; 51: 267-271.
- Tiscornia OM, Dreiling DA. ¿Regenera la glándula pancreática? GEN. 1966; 20: 657-664.
- Tiscornia OM, Dreiling DA. Recovery of pancreatic exocrine secretory capacity following prolonged ductal obstruction. Bicarbonate and amylase response to hormonal stimulation. Ann Surg.1966; 164: 267-270.
- Schlegel RD, Tiscornia OM, Vedia y Mitre E, Lembcye AR, Coqui R, Mecagno D, Cravino T, Waisman H. ¿Existe la regeneración pancreática? A.Ge.La. 2000;30:107-113.
Correspondencia: Fabiana López Mingorance
J E Uriburu 1044 – 1º piso. Dto 15. Ciudad Autónoma de Buenos Aires, Argentina
Tel: 4823-1843. Cel: 15 5404 9779
Correo electrónico: fabiannen@gmail.com
Acta Gastroenterol Latinoam 2016;46(4): 291-299
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE