María José Temido1 ID· Andrea Silva1 ID· Sandra Lópes1 ID· Ana Margarida Ferreira1 ID· Sofía Mendes1 ID· Manuela Ferreira1 ID· Pedro Figueiredo1,2 ID· Francisco Portela1,2 ID
1 Gastroenterology Department, Centro Hospitalar e Universitário de Coimbra.
2 Faculty of Medicine, University of Coimbra.
Coimbra, Portugal.
Acta Gastroenterol Latinoam 2024;54(1):56-64
Received: 10/11/2023 / Accepted: 19/02/2024 / Published online: 25/03/2024 /
https://doi.org/10.52787/agl.v54i1.364
Summary
Introduction. Recently, a subcutaneous formulation of infliximab was approved. The switch from intravenous infliximab to subcutaneous infliximab may improve convenience, but data on pharmacokinetics and immunogenicity are limited. We aimed to compare the efficacy and tolerability of subcutaneous infliximab and to assess whether the formulation results in higher plasma levels of infliximab. Methods. Retrospective, single-center cohort study. Patients with Crohn’s disease or ulcerative colitis, 18 years of age or older, clinically stable, receiving intravenous infliximab (5mg/kg every eight weeks) for more than 6 months were switched. Subcutaneous infliximab (120 mg) was administered every 2 weeks. Clinical and analytical evaluations were performed on day 0, week 2, week 8, and week 16. Plasma levels of infliximab and anti-drug antibodies were measured at these time points. Results. 41 patients included (27 with Crohn’s disease and 14 with ulcerative colitis. Azathioprine was administered concomitantly in 47.6%. All patients remained in clinical remission. Analytical biomarkers remained stable. Treatment persistence: 95.1%. Median plasma levels of infliximab at day 0, week 2, week 8, and week 16: 4.89 (3.25 – 7.27), 9.17 (7.89 – 12.6), 19.91 (15.02 – 21.64) and 21.55 (17.18 – 29.57) ug/mL, respectively. Statistically significant difference of plasma levels of infliximab in day 0 vs. week 2 and week 2 vs week 8. Azathioprine resulted in a statistically significant difference in plasma levels of infliximab only at day 0 (not at week 2, week 8, or week 16). Plasma levels of infliximab at week 2, week 8, and week 16, but not at baseline, were statistically associated with body mass index. No anti-drug antibodies were detected. No severe adverse effects. Three patients developed injection site reactions. Three patients developed arthralgia of undetermined significance. Conclusions. Switching from intravenous infliximab to subcutaneous infliximab maintained clinical response. Plasma levels of infliximab at week two were still lower than expected, considering the results at week eight. A possible advantage of subcutaneous infliximab may be the diminished necessity for concomitant immunomodulators.
Keywords. Crohn disease, ulcerative colitis, subcutaneous infliximab, intravenous infliximab, inflammatory bowel disease.
Del Infliximab intravenoso al subcutáneo: ¿igual pero diferente? Evidencia del mundo real de un centro terciario
Resumen
Introducción. Recientemente, se aprobó una formulación subcutánea de infliximab. El cambio de infliximab intravenoso a infliximab subcutáneo puede mejorar la comodidad de la administración, pero los datos sobre farmacocinética e inmunogenicidad son limitados. Nuestro objetivo fue comparar la eficacia y tolerabilidad de infliximab subcutáneo y evaluar si la formulación resultó en niveles plasmáticos más altos de infliximab. Métodos. Estudio retrospectivo de cohorte de un solo centro. Se realizó un cambio en el tratamiento de los pacientes con enfermedad de Crohn o colitis ulcerosa, mayores de 18 años, clínicamente estables, que recibían infliximab intravenoso (5 mg/kg cada 8 semanas) durante más de 6 meses. Se les administró cada dos semanas infliximab por vía subcutánea (120 mg). Se realizaron evaluaciones clínicas y analíticas en: día 0, semanas dos, ocho y 16. En esos momentos se midieron los niveles plasmáticos de infliximab y los anticuerpos antifármacos. Resultados. Fueron incluídos 41 pacientes (27 con enfermedad de Crohn y 14 con colitis ulcerosa). Al 47,6% se les administró concomitantemente azatioprina. Todos los pacientes mantuvieron la remisión clínica. Los biomarcadores analíticos se mantuvieron estables. Persistencia del tratamiento: 95,1%. La mediana de los niveles plasmáticos de infliximab en el día 0 y las semanas dos, ocho y 16 fue 4,89 (3,25 – 7,27), 9,17 (7,89 – 12,6), 19,91 (15,02 – 21,64) y 21,55 (17,18 – 29,57) μg/mL, respectivamente. Los niveles plasmáticos de infliximab presentaron una diferencia estadísticamente significatival en el día 0 vs. las semanas dos y ocho. La azatioprina condujo a una diferencia estadísticamente significativa en los niveles plasmáticos de infliximab solo en el día 0 (no en las semanas dos, ocho y 16). Los niveles plasmáticos de infliximab en las semanas dos, ocho y 16, no en el inicio, se asociaron estadísticamente con el índice de masa corporal. No se detectaron anticuerpos antifármacos. No hubo efectos adversos graves. Tres pacientes desarrollaron reacciones en el lugar de la inyección. Tres pacientes desarrollaron artralgia de significado indeterminado. Conclusiones. El cambio de infliximab intravenoso a infliximab subcutáneo mantuvo la respuesta clínica. Los niveles plasmáticos de infliximab en la semana dos todavía eran más bajos de lo esperado, considerando los resultados en la semana ocho. Una posible ventaja del infliximab subcutáneo puede ser la disminución de la necesidad de administrar inmunomoduladores concomitantes.
Palabras claves. Enfermedad de Crohn, colitis ulcerosa, infliximab subcutáneo, infliximab intravenoso, enfermedad inflamatoria intestinal.
Abbreviations
IBD: Inflammatory bowel disease.
IFX: Infliximab.
CD: Crohn’s disease.
UC: Ulcerative Colitis.
TNF: Tumor necrosis factor.
iv: Intravenous.
sc: Subcutaneous.
ivIFX: Intravenous Infliximab.
scIFX: Subcutaneous Infliximab.
IFXpl: Plasma levels of IFX.
SCCAI: Simple Clinical Colitis Activity Index.
HBI: Harvey-Bradshaw Index.
BMI: Body mass index.
FCP: Fecal calprotectin.
CRP: C-reactive protein.
Introduction
Inflammatory bowel disease (IBD) is one of the most common immune-modulated diseases worldwide, and its incidence is increasing.1 The pathophysiology and risk factors underlying this condition are still under debate, and widely effective therapies are yet to be discovered. Infliximab (IFX) is one of the most commonly used drugs for the treatment of Crohn’s disease (CD) and ulcerative colitis (UC). IFX is a chimeric anti-tumor necrosis factor (TNF) antibody. Many years after its introduction, this monoclonal antibody remains one of the most effective tools available for the treatment of these diseases.2 European and American guidelines recommend infliximab as first-line treatment for the induction and maintenance treatment of moderate to severe UC and CD.3-6
IFX has been administered as an intravenous (iv) formulation. Nonetheless, iv administration can have an impact on the quality of life of patients, as it indicates a dependence on hospital visits and a long infusion time. Additionally, iv drugs have created a huge demand for nurses and hospital facilities. Switching from an iv to a subcutaneous (sc) formulation has shown a tendency to increase patient convenience and satisfaction.7
In addition, to reduce the immunogenicity of IFX, an immunomodulator is recommended concomitantly with the iv formulation.3-6 This results in a higher risk of side effects and less convenience for patients, who not only have to take the injections, but also have to take the drug orally every day.
A new sc formulation of IFX (CT-P13 SC) was recently approved by the European Medicines Agency, initially in rheumatoid arthritis and IBD.8 The safety and efficacy of switching from intravenous Infliximab (ivIFX) to subcutaneous Infliximab (scIFX) has been evaluated,9-11 but studies of this transition in a real-world setting are still scarce. In addition, pharmacokinetic and immunogenicity data are limited.12 Iv administrations results in rapid and high peak plasma concentrations of the molecules. In contrast, sc biologics are absorbed more slowly, leading to lower and broader peak concentrations, resulting in more stable and homogenous concentrations over time.
Consequently, it has been hypothesized that this formulation may lead to less formation of anti-drug antibodies due to its unique pharmacokinetic properties.12
In fact, a recent randomized controlled trial evaluating the efficacy and safety of this new formulation showed that scIFX resulted in less anti-IFX antibodies formation than ivIFX.12
Thus, we aimed to assess the symptomatic and analytical efficacy and tolerability of scIFX. We also wanted to confirm whether this formulation resulted in higher plasma IFX levels (IFXpl) and to evaluate the clinical and analytical factors that may influence IFXpl in a real-world setting.
Materials and Methods
Ethical considerations
The project was conducted in accordance with good clinical practice and adhered to the ethical principles of the Declaration of Helsinki. All data were anonymized before the analysis. Written informed consent was obtained prior to inclusion in this study.
Study design
This retrospective single-center cohort study was conducted in a tertiary hospital in Portugal. The transition from iv to sc IFX was performed.
Patients were aged 18 years or older, with either UC or CD, undergoing maintenance therapy with ivIFX
(5 mg/kg every eight weeks) (without prior biologic therapy prior to infliximab) for more than 6 months, clinically stable (Simple Clinical Colitis Activity Index (SCCAI) < 3 or Harvey-Bradshaw Index (HBI) < 5), and normal C-reactive protein (CRP). In addition, all patients with prior anti-drug antibodies were excluded from the study. All patients who switched between September 2021 and July 2022 were included. The follow-up period was defined as the time from the first dose of scIFX to the end of December 2023.
Baseline information collected included age, age at diagnosis, weight, duration, extent and location of the disease, presence of perianal disease, time from diagnosis to the initiation of scIFX, history of prior abdominal surgery for IBD, concomitant use of immunomodulators or corticosteroids, body mass index (BMI), SCCAI and HBI, biomarkers (fecal calprotectin (FCP) and C-reactive protein (CRP), IFXpl, and anti-drug antibodies prior to scIFX initiation. We also evaluated the median IFXpl for the year preceding the transition. The concomitant use of immunomodulators was not discontinued during the study period. Categories of patients with respect to disease behavior were created according to the Montreal classification to homogenize patients’ subgroups characteristics.
ScIFX was administered at 120 mg every other week. The initial administration of scIFX coincided with the last intravenous dose. Clinical and analytical evaluations were performed on day 0 (d0) and at weeks two (w2), eight (w8), and 16 (w16) after initiation of treatment. The analytical evaluation included the assessment of CRP, FCP, hemoglobin, leukogram, albumin, alkaline phosphatase, alanine transaminase, and creatinine. One year after the initiation of scIFX, an additional clinical and analytical assessment of FCP was conducted.
IFXpl and anti-drug antibodies were also measured at these time points. This assessment was performed immediately before the next dose. The LISA-TRACKER kit from Theradiagnostics was used. Positive anti-drug antibodies were defined as those with a concentration higher than ≥ 10 AU/mL. Adverse events, discontinuations, and dose escalations were recorded.
Clinical remission was defined as SCCAI < 3 or HBI < 5, and analytical remission was defined as FCP
≤ 150ug/mg and CRP ≤ 1.5 mg/dL.
Outcomes
The primary outcome was the maintenance of clinical and analytical remission. Secondary outcomes included clinical and analytical factors associated with IFXpl and anti-drug antibodies, and the proportion of patients with adverse events or discontinuation of therapy.
Statistical analysis
Data were analyzed using Stata (StataCorp LP®) (version 16.0). Descriptive statistics were used to describe clinical and analytical data at baseline and throughout follow-up. Continuous variables were described as median and interquartile range (IQR), and categorical variables as frequencies. Comparisons of CRP, FCP, hemoglobin, albumin and IFXpl between time points were performed using the Wilcoxon signed-rank test. Univariate analysis of factors associated with IFXpl was performed using the Wilcoxon rank-sum test (categorical or binomial variables) and Spearman’s correlation with Bonferroni correction (continuous variables). Multivariate analysis of the factors associated with IFXpl was performed using median regression. Factors with a p < 0.2 in the univariate analysis and those that were clinically relevant were used for multivariable analysis. Statistical significance was set at p < 0.05.
Results
Patient characteristics
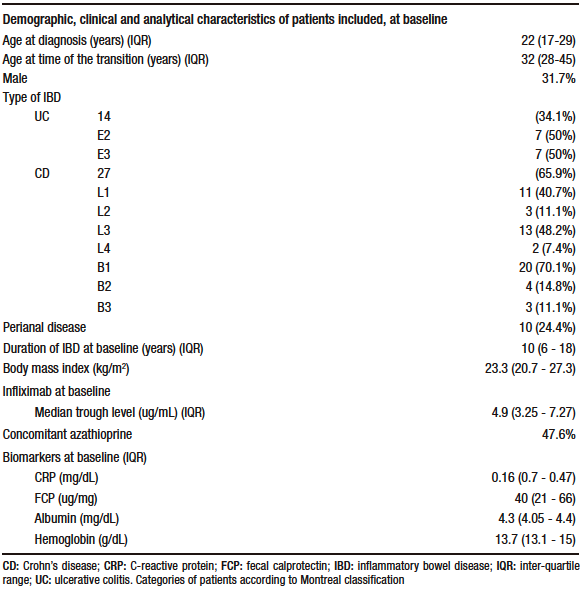
We included 41 patients, 31.7% of whom were male. CD was present in 27 (65.9%) patients and 14 (34.1%) had UC. The median age of the patients was 32 years (IQR 28-45). The median BMI was 23.3 (IQR 20.7-27.3). Before the transition, 47.6% of the patients were on concomitant immunomodulators, all of whom received azathioprine. The previously administered intravenous infliximab was a biosimilar. None of the patients were receiving concomitant corticosteroids. Demographic, clinical, and analytical characteristics of the patients at baseline are summarized in Table 1.
Table 1. Demographic, clinical and analytical characteristics of patients included at baseline (N = 41 patients) who underwent a transition from intravenous to subcutaneous infliximab
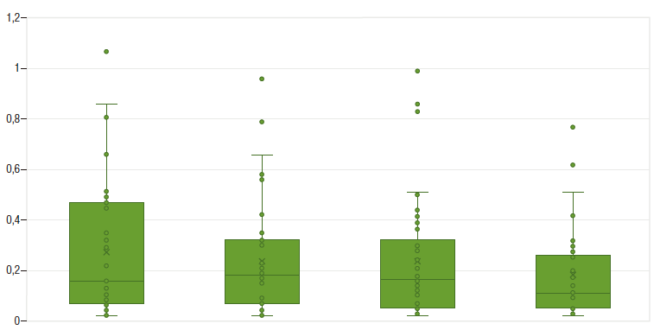
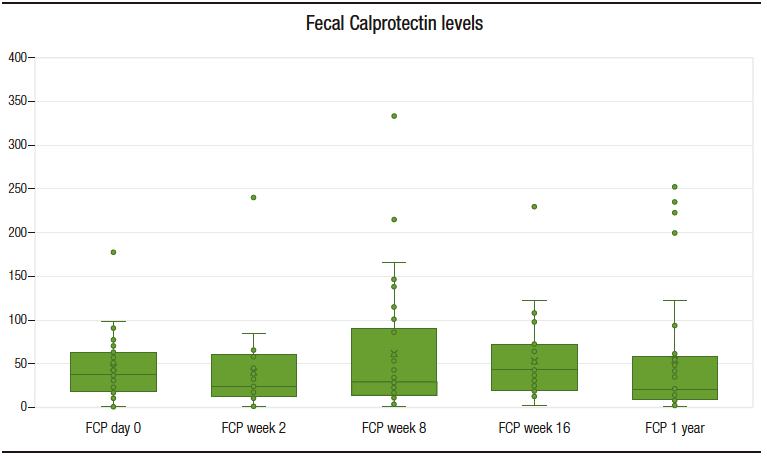
Median follow-up period was 25 months (IQR 22-26). All patients maintained clinical remission. CRP levels remanined approximately stable during the study period (Figure 1). With regard to FCP, only one patient developed a marked elevation of this biomarker (with posterior normalization), in contrast to the rest of the cohort, whose levels remained stable throughout the whole follow-up period, as shown in Figure 2.
Figure 1. Serum C-reactive protein levels throughout the study (evaluations at baseline, w 2, w 8 and w 16)

Figure 2. Fecal calprotectin serum level throughout the study (evaluations at baseline, w 2, w 8, w 16 and after one year of follow-up)
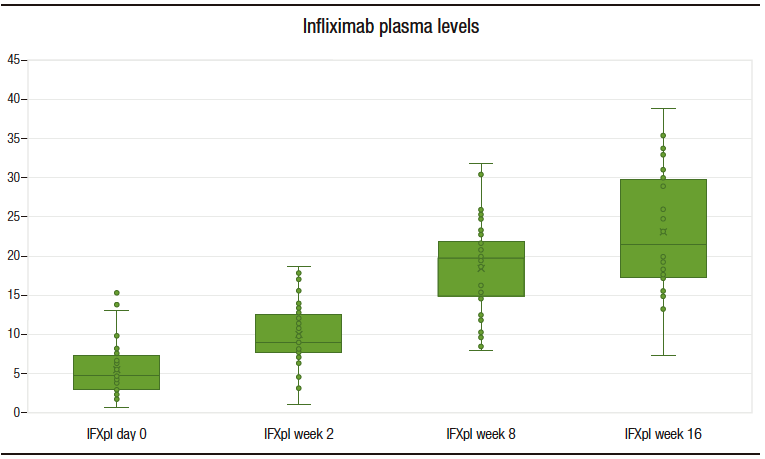
Pharmacokinetics of infliximab
The median IFXpl at d0, w2, w8 and w16 were 4.89 (3.25 – 7.27), 9.17 (7.89 – 12.6), 19.91 (15.02 – 21.64) and 21.55 (17.18 – 29.57) ug/mL, respectively. The difference between the median IFXpl in the year preceding the transition and IFXpl on d0 was not statistically significant. A statistically significant difference was found between the median IFXpl values at different time points (d0 vs. w2 and w2 vs. w8) (Figure 3). The difference between the IFXpl values at w8 and w16 was not statistically significant (p = 0.09).
No patient developed detectable anti-drug antibodies.
Figure 3. Infliximab plasma levels throughout the study (evaluations at baseline, w2, w 8 and w 16)
Effect of clinical and biochemical variables on infliximab plasma levels
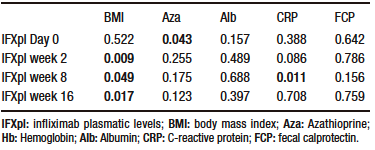
IFXpl measured at w 2, w 8, and w 16, but not at baseline, had a statistically significant association with BMI. Azathioprine showed a statistically significant association with IFXpl at day 0, but not at w 2, w 8, and weeks 16. No statistically significant association was found between IFXpl at different time points and type of IBD, age, sex, time from diagnosis to initiation of IFX, albumin or hemoglobin levels, FCP, and CRP (Table 2).
No statistically significant associations were found in the multivariable analysis.
Table 2. Association between infliximab plasmatic levels at different time points and azathioprine, body mass index and biomarkers in study population (N=41 patients) that underwent a transition from intravenous to subcutaneous Infliximab (Wilcoxon Sign rank test; statistical significance with p < 0,05)
Adverse events and treatment continuation
No serious adverse events were documented.
Regarding treatment persistence, two patients discontinued treatment during the follow-up: one patient returned to ivIFX by personal choice, and one patient had to switch to another biologic therapy (vedolizumab) due to an adverse event (arthralgias of undetermined significance). This represents a treatment continuation rate of 95.1%. No patients had to be dose escalated or switched to another biologic therapy due to loss of efficacy.
In terms of adverse events, three patients developed injection site reactions and three patients developed arthralgia of undetermined significance. As previously mentioned, in one case of arthralgia, the patient initiated treatment with vedolizumab; in the other cases, two patients with arthralgia and three patients with injection site reactions, the adverse events resolved spontaneously, and the patients continued to use scIFX.
Discussion
IFX is still the first-line therapy for moderate-to-severe CD and UC, both for induction and maintenance of remission.3-6 Recently, a new scIFX formulation was approved. This formulation has been associated with increased convenience and patient satisfaction.7 In our cohort, there was a high rate of treatment persistence, consistent with previous studies.13
The clinical efficacy, tolerability, and satisfaction with scIFX in patients with IBD have been reported.9-11,13,14 In our cohort, there were no cases of clinical relapse, and only one patient developed transitory biomarker elevations. Moreover, previous studies have shown that few patients develop anti-IFX antibodies.9,13,14 In our study, none of the patients developed anti-IFN antibodies. This supports the idea that the transition from IV to scIFX does not lead to a loss of efficacy or tolerability in IBD. However, the authors noted that the absence of relapse may be due to the strict selection of patients in this transition.
Pharmacokinetics differ depending on the route of administration. In scdrugs, the Ctrough (blood plasma concentration of the drug immediately before the next administration) is higher because plasma concentrations are more stable and do not reach high peak or low trough concentrations, unlike the pharmacokinetics of intravenous drugs. In fact, it has been confirmed that IFXpl in sc formulations can be measured at any time point during the cycle.15 In our patients, IFXpl levels were higher than those in iv formulations, consistent with previous studies.9,10 Nevertheless, the IFXpl on w2 was much lower than that on w8 and w16. From w2 to w8, there was a significant increase in IFXpl, and between w8 and w16, the levels were more stable with statistically significant differences. Consequently, the authors hypothesized that during the first week, the area under the curve is not sufficient for adequate exposure to IFX. This may lead to a loss of anti-drug antibodies response. The authors consider that the initiation of the sc formulation one or two weeks before the end of the ivIFX cycle would avoid a window of reduced drug exposure. This introduction may be considered for patients on higher doses or shorter intervals of ivIFX.
Azathioprine administered concomitantly with IFX induces higher rates of clinical remission than IFX monotherapy.4,16 These findings also apply to ivIFX. Nevertheless, combination therapy may lead to higher rates of mostly infectious adverse events.4,17 Regarding scIFX, it has been suggested that this formulation may be associated with reduced immunogenicity due to more stable plasma levels.12 In our study, we verified that azathioprine given concomitantly to IFX influence intravenous IFXpl, but not subcutaneous IFXpl. The authors suggest that scIFX may lead to improved pharmacokinetic properties and consequently increased plasma exposure to the drug. This may lead to a reduced need for immunomodulators, which may be an advantage of sc formulations.
However, it remains unclear whether scIFX doses should be individualized. Our group tried to identify the clinical and analytical factors that could potentially influence IFXpl. Inter-individual variation of IFXpl may be explained by body weight, but IFXpl modified by BMI has not been consistent across the studies.18 Buisson et al. investigated this association, but did not reach any conclusion possibly due to a low BMI in the majority of patients.9 In fact, in our study, higher BMI was statistically associated with lower IFXpl at the three time points of evaluation. The possible need for dose optimization in overweight patient needs to be considered in future prospective studies.
Our study revealed a treatment persistence rate of 95.1%, one of the highest compared to previous studies,13 which may be related to the high selection of our patients, which may have led to very good results in terms of efficacy.
Regarding adverse events, three patients in our cohort developed injection site reactions and three patients developed arthralgia of undetermined significance. One of these patients had a history of arthralgias that worsened after switching prior to scIFX initiation, leading to a change of biological agent in one patient. The remaining two patients had spontaneous resolution of this condition. None of the patients had detectable anti-IFX antibodies.
The main strengths of our study are as follows: a real-world analysis of a switch from intravenous to subcutaneous IFX, confirming its efficacy and safety. Moreover, in our study, we found that IFXpl of the sc formulation was not influenced by azathioprine, and a lower IFXpl was found in patients with a higher BMI. Finally, we found that levels were lower than expected on w2 compared to w8 and w16, which may result in inadequate exposure to the drug in this time window.
However, our study had several limitations. First, we did not evaluate the efficacy of transition in patients without a clinical or analytical remission. Patients with higher biomarkers may require dose escalation.9 In addition, we did not assess the efficacy of transition in patients receiving ivIFX > 5mg/kg and with less than eight weeks between administrations. Buisson et al. showed that these patients represent a population in which dose intensification may be required.9 Finally, this study should be continued over time with longer follow-up periods to assess all these variables and to see the need for drug optimisation as this study does not exceed the time in which most anti-TNFs would lose efficacy.
In the future, the authors see the need for studies with longer follow-up periods to assess the long-term efficacy and adherence to these subcutaneous formulations. In addition, studies are needed to evaluate the details of the pharmacokinetics of this drug and to identify patients in whom dose optimization would be beneficial.
Conclusion
In conclusion, switching from ivIFX to scIFX is a safe option in the maintenance treatment of UC and CD. ScIFX showed similar efficacy and tolerability compared to iv formulations and plasmatic levels of scIFX are influenced by BMI but not by concomitant azathioprine. Concomitant use of azathioprine may not be beneficial and patients with higher BMI may need optimized doses of scIFX.
Consent for Publication. Written informed consent was obtained from the patient or their parent, guardian, or relative to publish the data and/or clinical images for the benefit of science. A copy of the consent form is available to the editors of this journal.
Intellectual Property. The authors declare that the data, figures and tables that appear in this article are original and were made in their belonging institutions.
Funding. The author declares that there were no external sources of funding.
Conflict of Interest. Francisco Portela received speaker fees from Abbvie, Falk, Ferring, Janssen, Pfizer, Pharmakern, Takeda, and Tillotts. The other authors of this study report no conflict of interest.
Copyright
© 2024 Acta Gastroenterológica latinoamericana. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license, which allows non-commercial use, distribution, and reproduction, provided the original author and source are acknowledged.
Cite this article as: Temido M J, Silva A, Lópes S et al. From Intravenous to Subcutaneous Infliximab – The Same but Different? Real-world Evidence from a Tertiary Center. Acta Gastroenterol Latinoam. 2024;54(1):56-64. https://doi.org/10.52787/agl.v54i1.364
References
- Torres J, Mehandru S, Colombel J F, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741-55. https://DOI.org/10.1016/S0140-6736(16)31711-1
- Lasa J S, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2022;7:161-70. https://DOI.org/10.1016/S2468-1253(21)00377-0
- Raine T, Bonovas S, Burisch J, Torsten K, Bettenworth D, Chaparro M. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical treatment. J Crohn’s Colitis 2021;16:1-16. https://DOI.org/10.1093/ecco-jcc/jjab177
- Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO guidelines on therapeutics in Crohn’s disease: Medical treatment. J Crohn’s Colitis 2020;14:4-22. https://DOI.org/10.1093/ecco-jcc/jjz180
- Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol 2019;114:384-413. https://DOI.org/10.14309/ajg.0000000000000152
- Lichtenstein GR, Loftus E V, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am J Gastroenterol 2018;113:481-517. https://DOI.org/10.1038/ajg.2018.27
- Buisson A, Serrero M, Orsat L, et al. Comparative Acceptability of Therapeutic Maintenance Regimens in Patients With Inflammatory Bowel Disease: Results From the Nationwide ACCEPT2 Study. Inflamm Bowel Dis 2022:4-6. https://DOI.org/10.1093/ibd/izac119
- European Medicines Agency. Remsima (Infliximab): summary of opinion (post authorisation) 2020.
- Buisson A, Nachury M, Reymond M, et al. Effectiveness of Switching From Intravenous to Subcutaneous Infliximab in Patients With Inflammatory Bowel Diseases: the REMSWITCH
Study. Clin Gastroenterol Hepatol 2022. https://DOI.org/10.1016/j.cgh.2022.08.011 - Verma AM, Patel A, Subramanian S, Smith PJ. From intravenous to subcutaneous infliximab in patients with inflammatory bowel disease: a pandemic-driven initiative. Lancet Gastroenterol Hepatol 2021;6:88-9. https://DOI.org/10.1016/S2468-1253(20)30392-7
- Chaparro M, Garre A, Guerra Veloz MF, et al. Effectiveness and safety of the switch from remicade® to CT-P13 in patients with inflammatory bowel disease. J Crohn’s Colitis 2019;13:1380-6. https://DOI.org/10.1093/ecco-jcc/jjz070
- Schreiber S, Ben-Horin S, Leszczyszyn J, et al. Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology 2021;160:2340-53. https://DOI.org/10.1053/j.gastro.2021.02.068
- Smith PJ, Critchley L, Storey D, et al. Efficacy and Safety of Elective Switching from Intravenous to Subcutaneous Infliximab [CT-P13]: A Multicentre Cohort Study. J Crohns
Colitis 2022;16:1436-46. https://DOI.org/10.1093/ecco-jcc/jjac053 - Huguet JM, García-Lorenzo V, Martí L, et al. Subcutaneous Infliximab [CT-P13], a True Biologic 2.0. Real Clinical Practice Multicentre Study. Biomedicines 2022;10:1-11. https://DOI.org/10.3390/biomedicines10092130
- Roblin X, Veyrard P, Bastide L, Berger A, Barrau M. Subcutaneous injection of infliximab CT-P13 results in stable drug levels within 14-day treatment cycle in Crohn’s disease. Aliment Pharmacol Ther 2022;56:77-83;10.1111/apt.16852
- Colombel JF, Sandborn W, Reinisch W, et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N Engl J Med 2010;362:1383-95. https://DOI.org/10.1056/NEJMoa0904492
- Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of Serious and Opportunistic Infections Associated With Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018;155:337-346.e10. https://DOI.org/10.1053/j.gastro.2018.04.012
- Hanzel J, Bukkems LH, Gecse KB, D’Haens GR, Mathôt RAA. Population pharmacokinetics of subcutaneous infliximab CT-P13 in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 2021;54:1309-19. https://DOI.org/10.1111/apt.16609
Correspondence: María José Temido Mendes Ferreira
Email: mariajosetemido@gmail.com
Acta Gastroenterol Latinoam 2024;54(1):56-64
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE