Juan Lasa, Astrid Rausch, Ignacio Zubiaurre
Gastroenterology Department. Hospital Británico de Buenos Aires. Ciudad Autónoma de Buenos Aires, Argentina.
Acta Gastroenterol Latinoam 2018;48(2):106-116
Recibido: 15/05/2017 / Aprobado: 25/08/2017 / Publicado en www.actagastro.org el 18/06/2018
Summary
Background. Integrins are heterodimeric proteins that stimulate leukocyte adhesion to endothelial cells. Antibodies against integrins have been used as a therapeutic option in inflammatory bowel disease. Class-effect of these drugs has not been extensively assessed. Aim. To estimate the efficacy and safety of these drugs in inflammatory bowel disease. Material and methods. MEDLINE, EMBASE, LILACS and The Cochrane libraries were searched from 1966 to April 2017. Randomized, placebo-controlled trials in adults comparing anti-integrin antibodies versus placebo were eligible. Data was pooled to obtain relative risk of failure to achieve remission in active disease and relative risk of relapse of activity in quiescent disease, once remission had been achieved. Results. The search yielded 4201 citations, 10 of which were eligible. Anti-integrin antibodies were superior to placebo in inducing remission of both Crohn’s disease and ulcerative colitis [RR of no remission = 0.80 (0.73-0.87) and 0.86 (0.79-0.94), respectively]. They were superior to placebo in preventing relapse of Crohn’s disease [RR of relapse = 0.80 (0.73-0.87)]. One trial assessing anti-integrin antibodies efficacy in preventing relapse of Ulcerative colitis, showed that they were superior to placebo. Conclusion. Anti-integrin antibodies were superior to placebo in inducing and maintaining remission of active Crohn’s disease and Ulcerative Colitis.
Key words. Inflammatory bowel disease, integrins, biological therapy.
Eficacia y seguridad de los anticuerpos anti-integrina en la enfermedad inflamatoria intestinal: una revision sistematica y meta-analisis
Resumen
Introducción. Las integrinas son proteínas heterodiméricas que estimulan la adhesión leucocitaria al endotelio. Los anticuerpos anti-integrinas se han utilizado como alternativa terapéutica en la enfermedad inflamatoria intestinal. El efecto de clase de estas drogas no fue exhaustivamente evaluado. Objetivo. Estimar la eficacia y la seguridad de este tipo de drogas en la enfermedad inflamatoria intestinal. Material y métodos. Las bases de datos de MEDLINE, EMBASE, LILACS y Cochrane fueron revisadas desde 1966 hasta abril de 2017. Fueron seleccionados los ensayos aleatorizados y controlados con placebo en adultos comparando anticuerpos anti-integrinas versus placebo. Se buscó obtener el riesgo relativo del fallo en inducir la remisión en pacientes con enfermedad activa y el riesgo relativo del fallo en el mantenimiento de la remisión. Resultados. La búsqueda arrojó 4201 citas, de las cuales fueron 10 las utilizadas para el análisis. Los anticuerpos anti-integrina fueron superiores al placebo para inducir la remisión en la enfermedad de Crohn y la colitis ulcerosa [RR 0,80 (0,73-0,87) y 0,86 (0,79-0,94), respectivamente]. A su vez, mostraron ser superiores al placebo para el mantenimiento de la remisión en la enfermedad de Crohn [RR = 0,80 (0,73-0,87)]. Un estudio mostró que estos anticuerpos fueron superiores al placebo para el mantenimiento de la remisión en la colitis ulcerosa. Conclusiones. Los anticuerpos anti-integrinas demostraron ser superiores al placebo para la inducción y el mantenimiento de la remisión en enfermedad de Crohn y colitis ulcerosa.
Palabras claves. Enfermedad inflamatoria intestinal, integrinas, terapia biológica.
Abbreviations
CD: Crohn’s disease.
IBD: inflammatory bowel disease.
NNT: number necessary to treat.
PML: progressive multifocal leucoencephalopathy.
RR: relative risk.
UC: ulcerative colitis.
Inflammatory bowel disease (IBD) is a chronic disorder of the gastrointestinal tract of unknown etiology.1
There are two well-defined clinical entities of IBD: Crohn’s disease (CD) and ulcerative colitis (UC). These conditions carry a considerable morbidity, with an increased risk for hospital admissions, surgical treatment or even colorectal cancer.2
A wide variety of therapeutic options have been proposed, both pharmacological and surgical.3, 4 The investigation of the immunological mechanisms related to IBD has allowed the development of new therapeutic alternatives. Among these, antibodies against tumor necrosis factor alpha (TNF-a) have resulted in a significant impact on IBD’s natural history and prognosis.5 However, up to 30% of patients may not have and adequate response to these agents and approximately 50% may experience loss of efficacy during the first year of treatment.6
As a consequence, different monoclonal antibodies against other immunological mediators have been tested on IBD, such as anti-integrin antibodies. Integrins are heterodimeric proteins that stimulate leukocyte adhesion to endothelial cells.7 Thus; they play a key role in chronic inflammatory response. Alpha-4-beta-7 integrin is involved in the recruitment of leukocytes in the intestine. Natalizumab, an a4-integrin that acts against a4b1 and a4b7 integrin was first used on CD patients.8 Recently, a novel antibody directed against a4b7 integrin, Vedolizumab, was introduced, with promising results.9
Class-effect of these drugs on IBD has not been extensively assessed. Hence, we thought to perform a systematic review and meta-analysis of current evidence on this subject to assess their global efficacy as therapeutic agents against IBD. What is more, data on their safety is relevant, since Natalizumab8 has a restricted use due to the occurrence of potentially serious adverse events. Therefore, we also aimed to estimate the incidence of these events.
Materials and methods
Search strategy and study selection
A computer-based search of compatible papers from 1966 to April 2015 was performed using the following databases: MEDLINE-PubMed, EMBASE, LILACS and The Cochrane Library. Search strategy consisted of the following MESH terms: biologic therapy OR integrin OR leukocyte adhesion OR monoclonal antibody AND inflammatory bowel disease OR Crohn’s disease OR ulcerative colitis.
Relevant paper’s bibliographies were revised, as well as bibliographies from previously published meta-analyses. A manual search for potentially relevant abstracts from Digestive Disease Week and United European Gastroenterology Week from 2009-2016 was also undertaken.
Two authors performed bibliographic search in an independent manner. Potentially relevant abstracts were revised to check its inclusion. Inclusion criteria were: a) trials examining the efficacy of any anti-integrin antibody for IBD treatment; b) randomized, placebo-controlled trials; c) trials performed on adults. There were no language restrictions. Studies that implied simultaneous administration of anti-integrin antibodies and anti-TNF-a antibodies were excluded.
Search findings were then compared. If there was disagreement on the inclusion of a particular trial, it was discussed and determined by consensus. If there was evidence of duplication of data, the main author would be contacted to determine its inclusion.
Methodological evaluation of included studies
Methodological assessment was done using the Evidence-Based Gastroenterology Steering Group recommendations.10 A Jaded score of each trial was also calculated. If a significant difference in methodological quality among studies was observed, a sensitivity analysis would be undertaken by excluding those trials with less quality. If relevant data was missing in original manuscripts, authors would be contacted.
Outcome measures
The following outcomes were considered for analysis: efficacy of anti-integrin antibodies compared to placebo in terms of failure to achieve remission in active IBD and relapse of disease activity in quiescent IBD. Secondary outcomes included assessing the frequency of adverse events occurring as a result of therapy. Data were extracted as intention-to-treat analyses, in which all dropouts are assumed to be treatment failures, wherever trial reporting allowed this.
Statistical analysis
Meta-analysis was performed using REVMAN software (Review Manager Version 5.2. Copenhagen: The Nordic Cochrane Collaboration, 2012). Heterogeneity among studies was evaluated by means of chi square and I2 tests. A random-effect model was used to give a more conservative estimate of the effect of individual therapies, allowing for any heterogeneity among studies. Outcome measures were described as relative risk (RR) of failure to achieve remission and RR of relapse of disease activity in CD patients as well as in UC patients. Also, 95% confidence intervals were calculated. Funnel plots were designed to evaluate possible publication bias. Numbers necessary to treat (NNT) were calculated.
Results
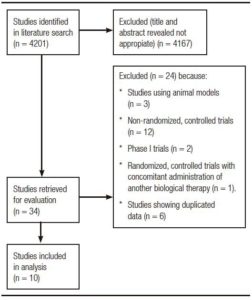
Search yielded 4201 bibliographic citations, 29 of which were identified as potentially relevant. Figure 1 describes reasons for exclusion of identified studies. Finally, ten randomized, placebo-controlled trials were included for analysis, which enrolled 4048 subjects.11-20
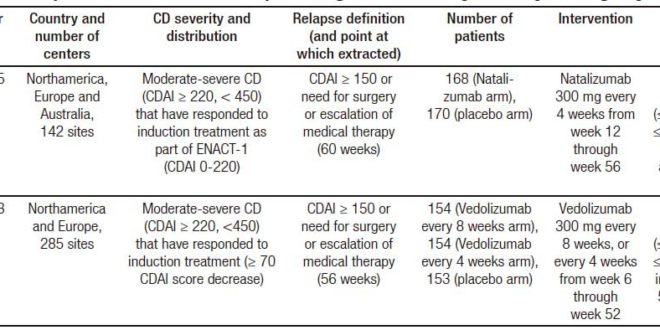
The characteristics of the included trials are described in Tables 1 (trials on induction of remission in CD patients), 2 (trials on relapse prevention in CD patients) and 3 (trials on induction of remission in UC patients). Clinical remission definitions as well as time of evaluation after intervention were similar among included studies.
Methodological evaluation of included trials is described in Table 4. No trial was excluded due to methodological limitations. Funnel plot is detailed in Figure 2, showing an asymmetry that suggests the presence of a potential publication bias.
Figure 1. Flow diagram of assessment of studies identified in the systematic review.
Efficacy of anti-integrin antibodies in inducing remission in CD
Four trials evaluating Natalizumab efficacy11-14 and three trials evaluating Vedolizumab efficacy15, 19, 20 were assessed. These trials enrolled 3408 patients. No significant heterogeneity was found among trials. Results are shown in Figure 3. Anti-integrin antibodies showed a significantly lower RR of failure to induce remission compared with placebo [RR 0.89 (0.83-0.94)], with a global NNT of 33. When considering trials that evaluated Vedolizumab, 1401 patients were analyzed. Vedolizumab also showed a significant lower RR of failure to induce remission [RR 0.86 (0.82-0.90)]. It is noteworthy that the trial published by Sands et al included patients with previous TNF antagonist failure. A sensitivity analysis was performed considering only anti-TNF-naïve patients and no significant differences were found on the outcome.
Table 1. Characteristics of randomized controlled trials of anti-integrin antibodies vs. placebo in inducing remission in active CD.
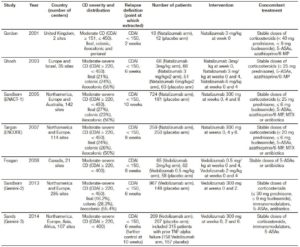
Table 2. Characteristics of randomized controlled trials of anti-integrin antibodies vs. placebo in preventing relapse in quiescent CD.

Table 3. Characteristics of randomized controlled trials of anti-integrin antibodies vs. placebo in inducing remission in active UC.
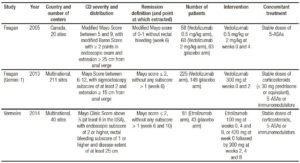
Table 4. Methodological evaluation of included randomized controlled trials.
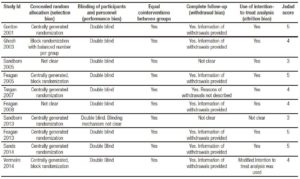
Figure 2. Funnel plot of included studies.
Efficacy of anti-integrin antibodies in inducing remission in UC
Two trials evaluating Vedolizumab efficacy on 555 patients16, 17 and one phase II trials assessing the efficacy of Etrolizumab18 were included. The trial published by Feagan et al in 2013 included a cohort of patients enrolled in an open-label group, which was not considered for analysis. No trials on Natalizumab were found on this particular subject. No significant heterogeneity among trials was found. Results are shown in Figure 4. Vedolizumab showed a significantly lower RR of failure to induce remission versus placebo [RR 0.85 (0.77- 0.94)], with a NNT of 8. Both trials assessed mucosal healing as an endpoint, though they used different scores (modified Baron Score and Mayo Score). When assessing Etrolizumab, the study by Vermeire et al showed that compared to placebo, Etrolizumab was significantly more effective in inducing clinical remission at week 10; however, more evidence is needed before drawing a valid conclusion since only a phase I and a phase II trial using Etrolizumab were published so far. Pooled results of Vedolizumab and Etrolizumab, as shown in Figure 4, still showed a significant efficacy of anti-integrin antibodies to induce remission. Once again, Vedolizumab showed a significantly lower RR of failure to induce mucosal healing versus placebo [RR 0.84 (0.74-0.94)]. Results are shown in Figure 5.
Figure 3. Forest plot of randomized controlled trials of anti-integrin antibodies versus placebo in inducing remission in active CD.
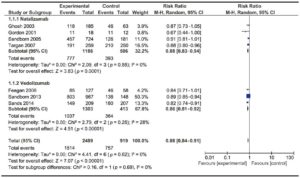
Figure 4. Forest plot of randomized controlled trials of anti-integrin antibodies versus placebo in inducing remission in active UC.
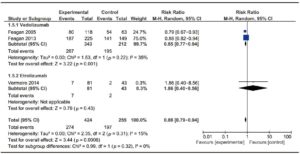
Figure 5. Forest plot of randomized controlled trials of anti-integrin antibodies versus placebo in inducing mucosal healing in active UC.
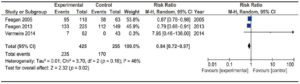
Efficacy of anti-integrin antibodies in preventing relapse in CD
Two trials assessed the efficacy of anti-integrin antibodies for relapse prevention in CD: one evaluating Natalizumab13 and the other evaluating Vedolizumab.19 Overall, they comprised 799 patients. No significant heterogeneity was found. Results are described in Figure 6. A significant difference was detected versus placebo [RR 0.80 (0.73-0.87)]. Global NNT was 11.
Efficacy of anti-integrin antibodies in preventing relapse in UC
Only one trial assessed Vedolizumab efficacy in preventing relapse in subjects with UC.17 A significantly higher efficacy for remission maintenance was found in those receiving Vedolizumab every 8 weeks (51/122, 41.8%) and every 4 weeks (56/125, 44.8%) than placebo (20/126, 15.9%) at 52 weeks (p < 0.001 in each case).
Figure 6. Forest plot of randomized controlled trials of anti-integrin antibodies versus placebo in preventing relapse in quiescent CD.
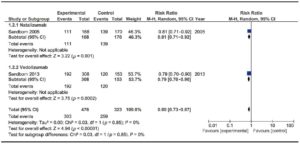
Adverse events
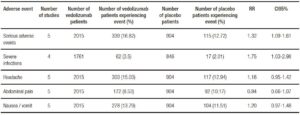
Tables 5 and 6 show adverse events incidence in patients treated with Natalizumab and Vedolizumab respectively versus placebo. Overall, no significant differences were found in terms of serious adverse events or serious infections when comparing Natalizumab versus placebo. Progressive Multifocal Leukoencephalopathy (PML) was not reported in any of the included studies. On the other hand, Vedolizumab showed an increased risk in serious adverse events and serious infections.
Table 5. Adverse events with Natalizumab vs. placebo in inducing remission in active CD.
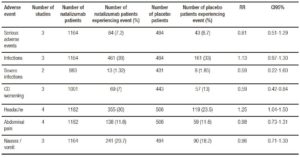
Table 6. Adverse events with Vedolizumab vs. placebo in inducing remission in active CD and UC.
Discussion
As specified by the results of our meta-analysis, there is a class-effect of anti-integrin antibodies for remission induction in patients with CD and UC. There is less evidence supporting their role for remission maintenance; in fact, available evidence failed to show a significant benefit for remission maintenance in patients with CD. According to our knowledge, this systematic review puts on perspective the utility of this kind of drugs suggesting that in the future new medicines that interfere leukocyte adhesion may represent a valid therapeutic alternative.
There is growing evidence regarding anti-TNF antibodies efficacy for induction and maintenance of clinical remission in CD and UC.21 However, a significant proportion of patients may not have a clinical response or lose response over time.22 Thus, there is a need for new therapeutic strategies in this area.
Integrin a4 inhibition has a proven effect on inflammatory response, not only on intestinal but also on extra-intestinal inflammation.8 Natalizumab inhibits a4ß7 and a1b1 integrins and is effective for CD treatment, as shown in previous meta-analysis by Ford et al. As a result of a1b1 inhibition, it has a significant effect on leukocyte adhesion in the central nervous system. In fact, it has been used as a therapeutic option for central nervous system autoimmune conditions, such as multiple sclerosis.24
Nevertheless, during the last few years, increasing reports on the development of PML caused by activation of JC virus in patients treated with Natalizumab have been published.25 As a consequence, its use has been restricted, resulting in the lack of further published experiences with this drug and the need for selective inhibition of a4b7 integrin.26 While Ertrolizumab27 and Vedolizumab have been developed, evidence of efficacy is strongest for Vedolizumab.
Vedolizumab is an IgG1 human antibody directed against a4b7 integrin that is effective for CD and UC in multicenter trials.17, 19, 20 According to our meta-analysis, Vedolizumab seems to have a better performance on UC than CD. More evidence is still required to determine the real magnitude of these differences. What is more, additional evidence is still required to evaluate the efficacy of this kind of drugs for maintenance of remission. It is worth mentioning that only one trial evaluated mucosal healing as an outcome measure.17 As a consequence, more evidence is still necessary on the efficacy of these drugs to induce and maintain mucosal healing. According to the trial published by Sands et al, Vedolizumab efficacy was greater when clinical outcomes were assessed at 10 weeks, after finishing induction therapy.20 This is an important point to be considered when conducting future clinical trials with anti-integrin antibodies, since remission should then be monitored a few weeks after induction therapy completion.
A rather promising therapeutic agent was also introduced in this systematic review: Etrolizumab, a humanized monoclonal antibody that selectively binds the b7 subunit of the heterodimeric integrins a4b7 and aEb7. There is very little evidence on its clinical efficacy, so caution must be taken when analyzing the results of the phase II clinical trial included in this systematic review.
Included trials with Vedolizumab have not shown any reported cases of PML. However, it is noteworthy that, unlike Natalizumab, Vedolizumab patients had a significant higher risk of serious adverse events and serious infections. This aspect highlights the need for further evidence assessing this aspect.
Finally, little evidence is shown on the efficacy of these drugs in patients who have already experienced TNF antagonist failure. Although anti-integrin antibodies would seem like a valid option in this clinical scenario, more evidence is still needed from prospective clinical trials.28
The main strength of this meta-analysis is that, it evaluates the class-effect of anti-leukocyte adhesion antibodies, instead of the individual effect of a single drug. This is relevant because it enforces the potential utility of future anti-leukocyte adhesion antibodies. Despite the rigorous search strategy, funnel plot asymmetry suggests the presence of publication bias that could be a significant limitation of this meta-analysis. We think, however, that this asymmetry may be due to the relatively scarce number of published trials. It is important to highlight that the results were expressed as a relative risk of failure to induce and/or maintain remission due to previously published meta-analyses such as the one published by Ford et al that use the same methodology.23
In conclusion, anti-integrin antibodies have shown a beneficial class effect for induction of clinical remission in patients with CD and UC. There is a need for more evidence on their efficacy for maintaining remission. Their role on IBD treatment still needs to be determined.
Authors’ contributions. JL performed the bibliographic search, undertook statistical analysis, contributed to the writing of the draft. AR performed the bibliographic search, contributed to the writing of the draft. IZ performed the bibliographic search, contributed to the writing of the draft, and reviewed the final draft.
Acknowledgements. We would like to thank to Dr. Michael Picco for the critical review made on this manuscript.
Conflicts of interest. Astrid Rausch is consultant physician for Takeda Pharmaceuticals. Ignacio Zubiaurre and Astrid Rausch are speakers for Abbvie Pharmaceutical Company. Ignacio Zubiaurre is consultant physician for Ferring Pharmaceuticals. Juan Lasa declares no conflict of interests.
References
- Abraham C, Cho JC. Inflammatory bowel disease. N Engl J Med 2009; 361: 2066-2078.
- Baars JE, Looman CW, Steyerberg EW, Beukers R, Tan AC, Weusten BL, Kuipers EJ, van der Woude CJ. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case-control study. Am J Gastroenterol 2011; 106: 319-328.
- Sica GS, Biancone L. Surgery for inflammatory bowel disease in the era of laparoscopy. World J Gastroenterol 2013; 19: 2445- 2448.
- Costa J, Magro F, Caldeira D, Alarcão J, Sousa R, Vaz-Carneiro A. Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease: A Systematic Review and Meta-analysis. Inflamm Bowel Dis 2013; 19: 2098-2110.
- Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 1997; 337: 1029-1035.
- Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol 2013; 108: 40-47.
- Cesarini M, Fiorino G. Leukocyte traffic control: a novel therapeutic strategy for inflammatory bowel disease – an update. Expert Rev Clin Immunol 2013; 9: 301-306.
- Keeley KA, Rivey MP, Allington DR. Natalizumab for the treatment of multiple sclerosis and Crohn’s disease. Ann Pharmacother 2005; 39: 1833-1843.
- Tilg H, Kaser A. Vedolizumab, a humanized mAb against the a4b7 integrin for the potential treatment of ulcerative colitis and Crohn’s disease. Curr Opin Investig Drugs 2010; 11: 1295-1304.
- Schoenfeld P, Cook D, Hamilton F, Laine L, Morgan D, Peterson W. An evidence-based approach to gastroenterology therapy. Gastroenterology 1998; 114: 1318-1325.
- Gordon FH, Lai CW, Hamilton MI, Allison MC, Srivastava ED, Fouweather MG, Donoghue S, Greenlees C, Subhani J, Amlot PL, Pounder RE. A randomized placebo-controlled trial of a humanized monoclonal antibody to alpha-4 integrin in active Crohn’s disease. Gastroenterology. 2001 Aug; 121 (2): 268-274.
- Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, Vyhnálek P, Zádorová Z, Palmer T, Donoghue S. Natalizumab Pan-European Study Group. Natalizumab for active Crohn’s disease. N Engl J Med 2003; 348: 24-32.
- Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S, van Deventer S, Goldblum R, Despain D, Hogge GS, Rutgeerts P. International Efficacy of Natalizumab as Active Crohn’s Therapy (ENACT-1) Trial Group. Evaluation of Natalizumab as Continuous Therapy (ENACT-2) Trial Group. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med 2005; 353: 1912-1925.
- Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, Spehlmann ME, Rutgeerts PJ, Tulassay Z, Volfova M, Wolf DC, Hernandez C, Bornstein J, Sandborn WJ. International Efficacy of Natalizumab in Crohn’s Disease Response and Remission (ENCORE) Trial Group. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology 2007; 132: 1672-1683.
- Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, Mc¬Donald JW, Cohen A, Bitton A, Baker J, Dubé R, Landau SB, Vandervoort MK, Parikh A. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha-4-beta-7 in¬tegrin. Clin Gastroenterol Hepatol 2008; 6: 1370-1377.
- Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, Mc¬Donald JW, Dubé R, Cohen A, Steinhart AH, Landau S, Aguzzi RA, Fox IH, Vandervoort MK. Treatment of ulcerative colitis with a humanized antibody to the alpha-4-beta-7 integrin. N Engl J Med 2005; 352: 2499-2507.
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A. GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699-710.
- Vermeire S, O’Byrne S, Keir M, Williams M, Lu TT, Mansfield JC, Lamb CA, Feagan BG, Panes J, Salas A, Baumgart DC, Sch¬reiber S, Dotan I, Sandborn WJ, Tew GK, Luca D, Tang MT, Diehl L, Eastham-Anderson J, De Herthogh G, Perrier C, Egen JG, Kirby JA, van Assche G, Rutgeerts P. Etrolizumab as induc¬tion therapy for ulcerative colitis: a randomized, controlled, phase 2 trial. Lancet 2014; 384: 309-318.
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A. GEMINI 2 Study Group. Vedolizumab as induction and maintenance thera¬py for Crohn’s disease. N Engl J Med. 2013; 369: 711-721.
- Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, D’Haens G, Ben-Horin S, Xu J, Rosario M, Fox I, Parikh A, Milch C, Hanauer S. Effects of Vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014; 147: 618- 627.
- Ehteshami-Afshar S, Nikfar S, Rezaie A, Abdollahi M. A systemat¬ic review and meta-analysis of the effects of infliximab on the rate of colectomy and post-operative complications in patients with inflammatory bowel disease. Arch Med Sci 2011; 7: 1000-1012.
- Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to inflix¬imab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol 2013; 108: 40-47.
- Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bow¬el disease: systematic review and meta-analysis. Am J Gastroen¬terol 2011; 106: 644-659.
- Rommer P, Dudesek A, Stüve O, Zettl U. Monoclonal Antibodies in Treatment of Multiple Sclerosis. Clin Exp Immunol 2014; 175: 373-384.
- Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012; 366: 1870-1880.
- Sakuraba A, Keyashian K, Correia C, Melek J, Cohen RD, Hanauer SB, Rubin DT. Natalizumab in Crohn’s disease: results from a US tertiary inflammatory bowel disease center. Inflamm Bowel Dis 2013; 19: 621-626.
- Rutgeerts PJ, Fedorak RN, Hommes DW, Sturm A, Baumgart DC, Bressler B, Schreiber S, Mansfield JC, Williams M, Tang M, Visich J, Wei X, Keir M, Luca D, Danilenko D, Egen J, O’Byrne S. A randomised phase I study of Etrolizumab (rhuMAb b7) in moderate to severe ulcerative colitis. Gut 2013; 62: 1122-1130.
- Lobaton T, Vermeire S, Van Assche G, Rutgeerts P. Review article: anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Ther 2014; 39: 579-594.
Correspondencia: Juan Lasa
Libertad 984 (ZIP Code: 1012). Ciudad Autónoma de Buenos Aires,
Argentina. Tel: (5411) 48234642 / Fax: (5411)48127944
Correo electrónico: drjuanslasa@gmail.com
Acta Gastroenterol Latinoam 2018;48(2): 106-116
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE