José M Menéndez, Leonardo Abramson, Raúl A Vera, Guillermo E Duza, Mariano Palermo
General Surgery and Gastrointestinal Division, Hospital Nacional Prof Alejandro Posadas, El Palomar, Provincia de Buenos Aires, Argentina.
Recibido: 13/06/2014 / Aprobado: 20/04/2015
Summary
Introduction. The ferric chloride intoxication is frequently caused by accident. Its toxicity is generally underrated, which can lead to fatal evolution or irreversible consequences. In this case, the caustic condition of the substance is related to the toxic properties of iron. Case presentation. A 36-year-old male patient arrives by ambulance indicating sensory deterioration. He presents erosive injuries in the buccal cavity and in the oropharynx, brownish teeth and metabolic acidosis. Toxicology tests and ferritin blood dosage are requested, which show a result from 1400 mg/dl. The symptoms are interpreted as acute iron intoxication. Due to the unfavorable evolution of his condition, an abdominal and pelvic CT scan are performed, which show extensive pneumoperitoneum and free fluid in the abdominal cavity. An exploratory laparotomy, a total gastrectomy with esophagostomy and feeding jejunostomy, washing and drainage due to perforated gastric necrosis caused by caustic ingestion are performed. Discussion. In our country, there is a high rate of intoxication caused by iron compounds, although it is not statistically measured. Nevertheless, the ferric chloride intoxication is extremely infrequent. The ingestion of this product leads to complications, which are associated with the iron concentration and its condition as a caustic agent. Conclusions. The surgical indications in the presence of intoxication caused by iron compounds are: stomach evacuation of iron, gastric necrosis, perforation or peritonitis and stenosis. Early or prophylactic gastrectomy is contraindicated. However, if complications that require immediate surgical intervention arise, there should be no hesitation and the corresponding procedure should be performed.
Key words. Gastrectomy, ferric chloride, intoxication.
Gastrectomía total por intoxicación con cloruro férrico
Resumen
Introducción. La intoxicación por cloruro férrico es frecuentemente causada de modo accidental. Su toxicidad está generalmente subestimada y puede devenir en un desenlace fatal o en secuelas irreversibles debido a que a las propiedades tóxicas del hierro se le asocia su condición de cáustico. Presentación del caso. Hombre de 36 años que ingresa a emergencias en ambulancia con deterioro del sensorio, lesiones erosivas en la cavidad bucal y orofaríngea, dientes parduzcos y acidosis metabólica. Se solicitan determinaciones toxicológicas y dosaje de ferritina sérica (1.400 mg/dl). Se interpreta el cuadro clínico como intoxicación aguda por hierro. Acorde a su evolución desfavorable, se realiza TC de abdomen y pelvis que evidencia extenso neumoperitoneo y líquido libre en cavidad abdominal. Se realiza una laparotomía exploradora, gastrectomía total, esofagostoma, yeyunostomía de alimentación, lavado y drenaje por necrosis gástrica perforada por la ingestión del cáustico. Discusión. En nuestro país, a pesar de carecer de estadísticas precisas, existe una elevada tasa de intoxicación por productos férricos. Sin embargo, la intoxicación por cloruro férrico es extremadamente infrecuente. La ingesta de este producto produce complicaciones asociadas a la concentración de hierro y su condición como agente cáustico. Conclusiones. Las indicaciones quirúrgicas en el caso de intoxicación por productos férricos son: evacuación gástrica del hierro, necrosis gástrica, perforación o peritonitis o estenosis. La gastrectomía precoz o profiláctica está contraindicada. No obstante, si surgen complicaciones que requieren inmediata intervención quirúrgica, no se debería vacilar y realizar el correspondiente procedimiento.
Palabras claves. Gastrectomía, cloruro férrico, intoxicación.
The iron intoxications (presented in its many forms) are frequently caused by accident. Its toxicity is underrated since it is a mineral supplement and its caustic action is unknown. This can lead to fatal evolution or irreversible consequences. The toxic action from iron does not only depend on its concentration but also on its pharmacological presentation. It can be classified into: mild intoxication 300 mg/dl; moderate intoxication 300 – 500 mg/ dl; severe intoxication > 500 mg/dl. In the case of ferric chloride, a substance used in electronics and at industrial level, the caustic condition of the substance is related to the toxic properties of iron. This is why the intoxication caused by ferric chloride produces erosion and corrosion of gastrointestinal mucosa, generating hemorrhagic gastroenteritis, which can lead to a perforation.
Clinical case presentation
A 36-year-old male patient with hypertension, DBT 2 and depression currently being treated arrives at the emergency room after suffering from sensory deterioration in a public space. According to the physical examination, the patient is hemodinamically stable, disoriented and afebrile. With nauseous and vomits and speaks incoherencies.
The patient presents positive bowel sounds, erosive injuries in the oral cavity and in the oropharynx, brownish teeth and oral mucosa. Blood test: HCT: 59, Hb 20, leukocytosis, hypertransaminasemia, hyperglycemia, acid-base status indicating normochloremic metabolic acidosis. The patient underwent with post-vomiting bronchoaspiration and black stools; for this reason, the patient is intubated and put on mechanical ventilation. Due to a consultation, the toxicology service requests regular toxicology tests, that shows negative results. Ferritin blood dosage is requested, which show a result from 1400 mg/dl. The symptoms are interpreted as acute iron intoxication. Chelation therapy with IV drip of deferoxamine 15mg/kg/hr during two hours is indicated. An abdominal x-ray is requested, which shows distended loops of bowel with no radiolucent images in the digestive tube; therefore, intoxication due to iron salts is dismissed. After interrogating patient’s family members, it is concluded that he ingested about 500 ml of ferric chloride, which is interpreted as a suicide attempt.
The patient underwent pre-renal acute renal failure; therefore, it is decided to perform a volume expansion with crystalloids, with further monitoring of the diuretic rhythm. The evolution of the patient is unfavorable, showing deterioration in regards of the initial symptoms, abdominal distension and intestinal paralysis; it is decided to transfer the patient to intermediate care unit. Ferritin blood test of 500 mg/dl.
During the evaluation, the patient still presents erosive injuries in the oral cavity and in the oropharynx, cervical subcutaneous emphysema, and distended and depressible abdomen, showing neither defense nor peritoneal reaction.
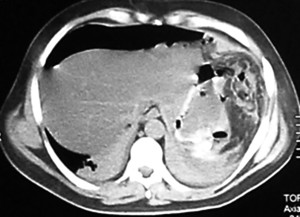
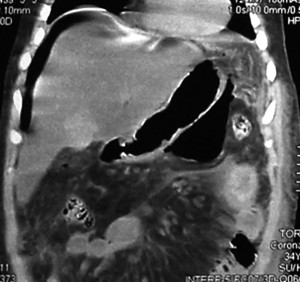
The patient underwent an upper digestive video-endoscopy, which produces a result of small-caliber esophagus, preserved mucosa and stomach with brownish-black mucosa spread diffusively all over the surface. No exploration of the digestive tube beyond the pylorus is performed, since there is a risk of perforation. Diagnosis: severe secondary gastric necrosis caused by caustic ingestion. Due to unfavorable progression, a thoracic, abdominal and pelvic CT scan was performed. The results showed extensive pneumoperitoneum, free liquid in the abdominal cavity and peri-gastric fat with signs of necrosis (Figures 1 A and B). In the presence of this image signs, a surgical approach is performed. The patient underwent a laparotomy, with total gastrectomy, esophagostomy and a jejunostomy for feeding, intensive wash and drainage of the abdominal cavity was done, due to perforated gastric necrosis caused by chloride ferric ingestion.
Figure 1. A y B. CT scan which demonstrates pneumoperitoneum, free fluid in the abdominal cavity and peri-gastric fat with signs of necrosis.
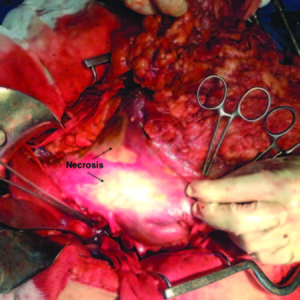
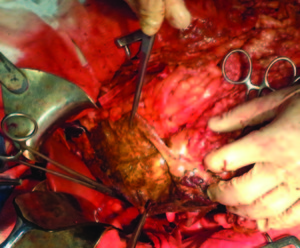
The intraoperative findings were localized necrosis observed in the lesser curvature and in the anterior wall of the stomach (Figure 2). Then, the stomach was opened and extense necrosis of the mucosa’s stomach was demonstrated (Figure 3). The patient was taken to intensive care unit for immediate postoperative care. He underwent surgery 24 hours after the ingestion and presented a hemoperitoneum caused by bleeding. His follow up in the postoperative days was remarkable, with deterioration in his clinical parameters. On the fourth day after the total gastrectomy was performed, a reoperation was done in order to do a second look. The presence of a duodenal stump dehiscence associated with a purulent collection in the left upper quadrant was found. The dehiscence was sutured and the collection was drained. After tis reoperation, the patient’s surgical follow up was unremarkable. On the 8th postoperative day, the patient died product of a systemic inflammatory response syndrome (SIRS) due to respiratory complications in intensive care unit.
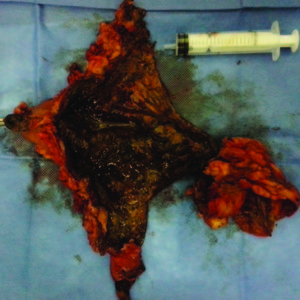
The macroscopic and pathology results showed intense coagulating necrosis (Figure 4).
Figure 2. Intraoperative: Localized necrosis is observed in the lesser curvature and in the anterior wall of the stomach.
Figure 3. Necrosis of the mucosa’s stomach.
Figure 4. Specimen: Necrosis caused by ferric chloride.
Discussion
Ferric chloride (FeCl3) is an acidic yellow-green or dark brown substance (pH 1.0). While iron salts toxicity is well documented, as regards to intoxication with ferric chloride the reported cases are rare.
Ferric chloride poisoning has high mortality risk. It is imperative for physicians to have clinical suspicion and handle the case without delay with aggressive measures. Detailed clinical interview, clinical suspicion and early diagnosis are treatments. Despite being a rare entity, it may provoke devastating effects in intentional ingestions. Intake of about 20-60 mg / kg of elemental iron is toxic, superior to 60 mg / kg of elemental iron could be fatal.
Iron peak in blood is a sensitive and specific indicator of iron poisoning. The mortality associated with serum iron in blood, especially if > 1000 μg/dl.
Unlike iron salts in which the intake ratio of absorption is near 30%, ferric chloride produces direct endothelial damage and gastrointestinal mucosal damage and consequently increased iron absorption.
Due to its acidic corrosive nature and content of elemental iron, FeCl3 causes both direct corrosive effect as well as cellular dysfunction resulting in severe gastrointestinal injury, metabolic acidosis, coagulopathy and shock. Moreover, it triggers thrombosis and protein binding and coagulation.
Iron and iron salts are erratically absorbed in the gastrointestinal tract mostly in ferrous state. Un-bound iron ion is unstable and toxic to vital tissues. The iron absorbed causes cellular dysfunction with resultant lactic acidosis and necrosis. The cellular mechanism is based on iron catalyst with production of free oxygen radicals that oxidize DNA, lipids, proteins and other biomolecules. It may cause endothelial damage and arterial thrombosis according to experimental studies in mice.
The ferric chloride toxicity results from direct corrosive effect and cellular dysfunction that may result in severe gastrointestinal damage, metabolic acidosis, CNS depression, liver dysfunction, coagulopathy, hypotension and shock. Vomiting is a sensitive but not specific sign. Severe vomiting associated with hematemesis are suggestive of intoxication.
The symptoms may induce severe alterations including severe hyperglycemia, metabolic acidosis, severe coagulopathy by consumption, liver failure, direct interference of Fe in coagulation cascade on vitamin K-dependent factors, decreased production of thrombin, inhibition of fibrinogen and even acute hemolysis.
The general treatment includes: immediate dilution, mouthwash, gastric lavage, advanced life support measures, management of acidosis and early endoscopy. Promptness of action is important to prevent iron absorption in the gastrointestinal tract. Induced vomit is not recommended and activated carbon has no effect on iron absorption.
Specific treatment consists in chelation with deferoxamine which is the gold-standard chelator in iron toxicity eliminating potential iron binding sites. The recommendations for deferoxamine include: coma, shock, seizures, metabolic acidosis, CNS depression, hepatotoxicity, serum iron > 500 mg/dl or > 350 mg/dl symptomatic.
In our country, there is a high rate of intoxication caused by iron compounds (especially in pediatric population), but it is not statistically measured. Nevertheless, the ferric chloride intoxication is extremely infrequent since it is used exclusively for photoengraving, photography, and in the manufacture of other iron salts, pigments, and ink; as a catalyst in organic reactions; in purifying factory effluents and deodorizing sweat; in the chlorination of silver and copper ores; as a mordant in dyeing and printing textiles; as an oxidizing agent in dye manufacturing and as an etching agent for printed circuitry. There are few reports of cases around the world, not having established the corresponding toxic dose. The ingestion of this product leads to complications, which are associated with the iron concentration and its condition as a caustic agent.
The clinical case shows the following phases:
1) Phase I (first 2 hs): vomiting, abdominal pain, diarrhea, hematemesis, lethargy, shock, hematochezia, acidosis and coagulopathy.
2) Phase II (after phase I): apparent recovery may contribute to a false sense of security.
3) Phase III (2 to 12 hours after phase I): profound shock, severe acidosis, cyanosis and fever.
4) Phase IV (2 to 4 days): possible hepatotoxicity.
5) Phase V (days to weeks): gastro-intestinal scarring; strictures.
Oral ingestion may produce mild to moderately severe oral and esophageal burns with more severe burns occurring in the stomach. Perforations are rare but may occur. The pyloric end of the stomach is most severely affected and is the site of delayed stricture occurring generally at 3 weeks after the ingestion.
Conclusions
- The surgical indications in the presence of intoxication caused by iron compounds are: stomach evacuation of iron, gastric necrosis with subsequent stomach retraction, and perforation or peritonitis and stenosis, having the possibility of performing gastrostomy, gastrostomy and gastric washing, and total or partial gastrectomy, according to the circumstantial complication
- Early or prophylactic gastrectomy is contraindicated, since there is no immediate structural damage and there is high risk of morbi-mortality if the procedure if performed in the context of an acute clinical case. However, as in this case, if complications that require immediate surgical intervention arise, there should be no hesitation and the corresponding procedure should be performed.
Referencias
- Wu ML, Yang CC, Ger J, Deng JF. A fatal case of acute ferric chloride poisoning. Vet Hum Toxicol 1998; 40: 31-34.
- Chen MR, Lin JL, Liaw SJ, Bullard MJ. Acute iron intoxication: a case report with ferric chloride ingestion. Zhonghua Yi Xue Za Zhi (Taipei) 1993; 52: 269-272.
- Mann KV, Picciotti MA, Spevack TA, Durbin DR. Management of acute iron overdose. Clin Pharm 1989; 8: 428-440.
- Wu ML, Tsai WJ, Ger J, Deng JF. Clinical experience of acute ferric chloride poisoning. Vet Hum Toxicol 2003; 45: 243-246.
- McGuigan MA. Acute iron poisoning. Pediatr Ann 1996; 25: 33-38.
- Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood 2013; 121: 3733-3741. doi: 10.1182/ blood-2012-11-468983. Epub 2013 Jan 23.
- Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res 1990; 60: 269-280.
- Wang X, Xu L. An optimized murine model of ferric chloride-induced arterial thrombosis for thrombosis research. Thromb Res 2005; 115: 95-100.
- Willhite CC, Ball GL, Bhat VS. Emergency do not consume/do not use concentrations for ferric chloride in drinking water. Hum Exp Toxicol 2013; 32: 260-74. doi: 10.1177/0960327112459208. Epub 2012 Oct 30.
Correspondencia: Mariano Palermo MAAC FACS
Av Pte Perón 10298, Ituzaingó, Buenos Aires, Argentina.
Correo electrónico: palermomd@gmail.com
Acta Gastroenterol Latinoam 2015;45(3):212-216
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE