Fernando Gruz,1 María Silvina Munné,2 Jorge González,2 María Aldana Lizarraga Villagran,3 Pablo Raffaele,3 Valeria Inés Descalzi1
1 Hepatology and Liver Transplant Unit. University Hospital, Fundación Favaloro. Ciudad Autónoma de Buenos Aires, Argentina.
2 National Reference Laboratory of Viral Hepatitis. National Institute of Infectious Diseases “Carlos G Malbran”. Ciudad Autónoma de Buenos Aires, Argentina.
3 Renal Transplant Unit. University Hospital, Fundación Favaloro. Ciudad Autónoma de Buenos Aires, Argentina.
Acta Gastroenterol Latinoam 2016;46:122-125
Recibido: 22/12/2015 / Aprobado: 04/03/2016 / Publicado en www.actagastro.org el 04/07/2016
Summary
We describe a clinical case of a kidney transplant patient who presented a sudden elevation of his liver function tests. Once we ruled out the most frequent causes of acute hepatitis, serum tests for Hepatitis E were performed. Hepatitis E virus RNA was detected in blood and stools. After six months the virus was still detected. Ribavirin treatment was initiated with normalization of the serum aminotransferases and sustained virology response was achieved.
Key words. Hepatitis E Virus, HEV, Chronic Hepatitis E, Ribavirin.
Tratamiento con Ribavirina de un paciente con hepatitis E crónica. Primer reporte en América Latina
Resumen
Se describe el caso de un paciente con antecedentes de inmunosupresión por trasplante renal, con alteración del hepatograma en el post-trasplante. Luego de haber descartado las causas más frecuentes, se solicitó la detección del RNA del virus E de la hepatitis en suero y materia fecal, los cuales fueron positivos. El paciente evolucionó a la cronicidad y se decidió tratarlo con Ribavirina, alcanzando la negativización una respuesta virológica sostenida.
Palabras claves. Virus E, hepatitis E, HEV, hepatitis crónica E, Ribavirina.
Abbreviations
CMV: Cytomegalovirus.
HAV: Hepatitis A virus.
HBV: Hepatitis B virus.
HCV: Hepatitis C virus.
ASMA: Anti Smooth Muscle Antibody.
Anti LKM1: Anti Liver-Kidney 1 Antibody.
ANA: Antinuclear Antibody.
HEV: Hepatitis E virus.
AST: Aspartate aminotransferase.
ALT: Alanine aminotransferase.
RNA: Ribonucleic Acid.
RBV: Ribavirin.
HIV: Human Immunodeficiency Virus.
Peg-IFN: Pegylated interferón.
Case Report
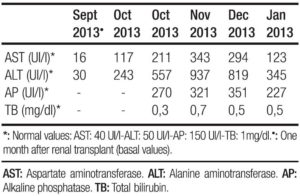
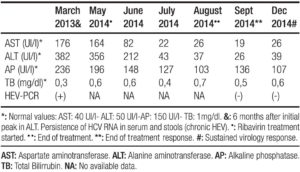
A 42 year old man with history of chronic renal failure and renal transplantation in June 2013, assisted to the liver team consulting room. He was referred by his nephrologist in October 2013, because of a sudden elevation of his liver function tests. He received a cytomegalovirus (CMV) mismatch cadaveric kidney graft (Receptor: CMV negative – Donor: CMV positive). Timoglobuline and steroids were the immunosuppressive therapy selected for induction, and tacrolimus, sodium mycophenolate and steroids, for maintenance. Other drugs that the patient also received were: omeprazole, valganciclovir, ferrous sulphate, calcium, nystatin, enalapril, thrimetoprim/ sulfametoxazole and magnesium citrate. The patient was discharged from the hospital twelve days after transplantation. He lived nearby Buenos Aires (capital city), but approximately one month after discharge travelled to a coastal city. No consumption of raw or undercooked meat or pork products occurred, but recreational use of sea water and shellfish intake was referred. In October 2013 a 3-4 fold increase in serum aminotransferases was noted and a week later they raised upto 18 times the upper normal value (Table 1). Total bilirrubin level and prothrombin concentration were normal, and no other laboratory abnormality was present. The physical exam was unremarkable, as well as the abdominal doppler ultrasound. Every possible hepatotoxic drug was discontinued and we ruled out the presence of HAV IgM, HBsAg, HBV IgMcore, HCV RNA, viral load for CMV, ASMA, Anti LKM- 1 and ANA. Serum immunoglobulins; ceruloplasmin, serum ferritin and transferrin saturation were within normal values. In spite of being an immunosuppressed patient HBV DNA was not done. Serum tests for hepatitis E virus (HEV) were performed: anti HEV (IgM) was negative, but HEV RNA was detected in serum and stools (genotype 3). Immunosuppressant therapy was switched to sirolimus (with low target levels) and steroids doses were diminished. During the follow up aspartate aminotransferase (AST) and alanine aminotransferase (ALT) persisted elevated (3 and 7 times higher than the upper normal limit, respectively) (Table 2). Six months after the initial peak in serum aminotransferases, tests for HEV were performed again, proving the persistence of the virus and leading us to the diagnosis of chronic hepatitis E. We performed a liver biopsy which only showed mild inflammation in the lobule, normal portal triads and no fibrosis. Ribavirin (RBV) 1000mg per day was started (May 2014) for sixteen weeks. In the seventh week of treatment ALT and AST returned to normal values and persisted so until end of follow-up (March 2015). New HEV RNA in serum and stools was undetectable at this time (October 2014: end of treatment response) and remained negative at twelve weeks after RBV was finished (sustained virologic response) (Table 2). Liver function tests persisted within normal values. No graft rejection was observed.
Discusión
Hepatitis E virus (HEV) is a member of the genus Hepeviridae. This non-enveloped single stranded RNA has four major genotypes (1 to 4), and two major species were described: avian and mammalian. It was first recognised during an epidemic of hepatitis in India in 1978.1 HEV infection is transmitted by the fecal–oral route. HEV genotypes 1 and 2 are the cause of more than 50% of epidemic cases of viral hepatitis in developing countries, whereas genotypes 3 and 4 have been found in several animal species (especially domestic swine, wild boar and deers), and they are responsible for autochthonous hepatitis E in industrialized countries.
Clinical features of HEV infection range from asymptomatic to acute hepatitis and even to liver failure.2 Symptomatic cases are characterized by jaundice, asthenia, fever, joint and muscle pain, hepatosplenomegaly and loss of appetite. These symptoms are usually self-limiting and resolve in 4–6 weeks. Atypical non-hepatic manifestations such as acute pancreatitis, haematological abnormalities, autoimmune phenomena, Guillan Barre syndrome and other neurological syndromes have also been reported.3, 4 Some patients like pregnant women and individuals with chronic liver diseases and superimposed HEV infection have a severe course. In general population HEV mortality rate has been reported to be about 1% whereas in pregnant women is as high as 30%.5
Chronic hepatitis E has been reported among immunosuppressed persons, in particular in HIV (+) patients, in patients with haematological malignancies and in solid organ transplant recipients.4 Most patients with chronic evolution of HEV infection are asymptomatic and only elevations of liver function tests are observed.3 It has been reported a rapid evolution to cirrhosis after kidney, kidney-pancreas and liver transplantation.6, 7
Neither guidelines nor accepted treatments exist for chronic hepatitis E, and therapeutic options go from reduction of the immunosuppressant regime, which has been related to spontaneous clearance of the virus in 30% of patients,8 to antiviral agents, such as RBV monotherapy9, 10 or Pegylated interferon (Peg-IFN) with or without RBV.11 It has been described that Peg-IFN therapy could be unsafe in kidney transplant patients as it is associated with graft rejection and renal failure.12 RBV has a broad-spectrum against many RNA viruses and it has been used for chronic HEV treatment with good results, inducing sustained virologic response in approximately 80% of cases.13, 14 Doses vary from 200 to 1200 mg/day.15, 16 We decided to treat our patient with a relatively high dose (12 mg/Kg: patient’s body weigh 83 kg) without evidence of anemia during treatment. Despite of the efficacy of a short treatment period (12 weeks) being well described, we decided to treat our patient for a longer period (16 weeks). The rational for this was based on some reports of virus relapse with a 12-week treatment.10
Tests for HEV are not routinely done to the donors in Argentina, so we cannot exclude that the patient might have been infected during the transplant.
To the best of our knowledge this is the first case reported of treatment with RBV in a patient with chronic HEV in Latin America. We decided to switch the immunosuppressant regime from Tacrolimus to Sirolimus as the former has been described as an independent predictive factor for chronicity17 and we also decreased steroids doses. As no response was observed after six months from the time of the HEV diagnosis, we started treatment with RBV monotherapy for sixteen weeks. No anemia was noted and liver function tests returned to normal values rapidly. At the end of treatment HEV was undetectable in the serum and stools and remained negative at twelve weeks after having stopped RBV. Unfortunately, we could not perform HEV tests during the treatment in order to be aware of the moment that HEV-RNA became undetectable. HEV has to be part of the routine exams for patients with abnormal liver function tests.
Chronic infection has to be treated in order to avoid advance liver fibrosis. RBV monotherapy is a useful and safe option.
Conclusions
We have reported the first case in Latin America of chronic hepatitis E in a kidney transplant recipient. Although Argentina is considered a low endemic country for hepatitis E, we believe that its incidence is underestimated. Chronic HEV must be suspected in immunosuppressed patients with liver function tests disturbance. It has to be treated in order to avoid advanced liver fibrosis. RBV monotherapy is a useful and safe option and may induce sustain virology response.
Financial support. It hasn´t received financial support.
References
- Khuroo MS. Study of an Epidemic of Non-A, Non-B Hepatitis Possibility of Another Human Hepatitis Virus Distinct from Post-Transfusion Non-A, Non-B Type. 1980 The Am J Med 1980; 68: 818-824.
- Scobie L, Dalton HR. Hepatitis E: source and route of infection, clinical manifestations and new developments. J Viral Hepat 2013; 20: 1-11.
- Aggarwal R. Clinical presentation of hepatitis E. Virus Res 2011; 161: 15-22.
- Fujiwara S, Yokokawa Y, Morino K, Hayasaka K, Kawabata M, Shimizu T. Chronic hepatitis E: a review of the literature. Journal of Viral Hepatitis 2014; 21: 78-89.
- Kumar A, Beniwal M, Kar P, Sharma JB, Murthy NS. Hepatitis E in pregnancy. Int Gynaecol Obstet 2004; 85: 240-244.
- Gerolami R, Moal V, Colson P. Chronic Hepatitis E with cirrhosis in a Kidney Transplant Recipient. N Engl J Med 2009; 361: 1025-1027.
- Haagsma EB, van den Berg AP, Porte RJ et al. Chronic Hepatitis E Virus Infection in Liver Transplant Recipient. Liver Transplant 2008; 14: 574-553.
- Suneetha PV, Pischke S, Schlaphoff, V et al. Hepatitis E virus (HEV)-specific T-cell responses are associated with control of HEV infection. Hepatology 2012; 55: 695-708.
- Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D’Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso AM, Abravanel F, Pol S, Rostaing L, Mallet V. Ribavirin for Chronic Hepatitis E Virus Infection in Transplant Recipients. NEJM 2014; 370; 1111-1120.
- Kamar N, Rostaing L, Abravanel F, Garrouste C, Lhomme S, Espósito L, Basse G, Cointault O, Ribes D, Nogier MB, Alric L, Peron JM, Izopet J. Ribavirin Therapy Inhibits Viral Replication on Patients With Chronic Hepatitis E Virus Infection. Gastroenterology 2010; 139: 1612-1618.
- Kamar N, Abravanel F, Garrouste C, Cardeau-Desangles I, Mansuy JM, Weclawiak H, Izopet J, Rostaing L. Three-month pegylated interferon-alpha-2a therapy for chronic hepatitis E virus infection in a haemodialysis patient. Nephrol Dial Transplant 2010; 25: 2792-2795.
- Rostaing L, Izopet J, Baron E, Duffaut M, Puel J, Durand D. Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation 1995; 59: 1426-1431.
- Kamar N, Rostaing L, Abravanel F, Garrouste C, Lhomme S, Espósito L, Basse G, Cointault O, Ribes D, Nogier MB, Alric L, Peron JM, Izopet J. Ribavirin Therapy Inhibits Viral Replication on Patients With Chronic Hepatitis E Virus Infection. Gastroenterology 2010; 139: 1612-1618.
- Mallet V, Nicand E, Sultanik P, Chakvetadze C, Tesse S, Thervet E, Mouthon L, Sogni P, Pol S. Brief Communication: Case Reports of Ribavirin Treatment for Chronic Hepatitis E. Annals of Internal Medicine 2010; 153: 2.
- Peters van Ton AM, Gevers TJG, Drenth PH. Antiviral Therapy in Chronic Hepatitis E: A Systematic Review. Journal of Viral Hepatitis 2015; 25: 965-973.
- Dalton H, Pas S, Madden R, van der Eijk A. Hepatitis E virus: Current Concepts and Future Perspectives. Curr Infect Dis Rep 2014; 16: 399.
- Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011; 140: 1481-1489.
Correspondencia: Fernando Gruz
Av Belgrano 1782, Piso 7. Unidad de Hepatología y Trasplante Hepático. Ciudad Autónoma de Buenos Aires, Argentina
Tel.: +541143781366
Fax: +541143781392
Correo electrónico: fgruz@ffavaloro.org
Acta Gastroenterol Latinoam 2016;46(2):122-125
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE