Rafael Vaz Pandini, Sérgio Silveira Júnior, Rodrigo Ambar Pinto, Caio Sérgio Rizkallah Nahas, Francisco Tustumi, Rafaela Brito Bezerra Pinheiro, Ulysses Ribeiro Júnior, Sérgio Carlos Nahas, Ivan Cecconello
Hospital das Clínicas, Gastroenterology Department, University of São Paulo, São Paulo, Brazil.
Acta Gastroenterol Latinoam 2020;50(2):154-158
Recibido: 06/08/2018 / Aprobado: 27/07/2019 / Publicado en www.actagastro.org el 29/06/2020
Summary
Gastrointestinal stromal tumours (GIST) are the most common neoplasms arising from the mesenchymal cells of the gastrointestinal tract. Although GIST can arise in any gastrointestinal site, the most common are stomach and small bowel. Less than 5% of all GIST are located in rectum. Surgery is the main treatment option for resectable GIST. Currently, local excision has been increasingly employed thanks to neoadjuvant therapy. This paper reports two cases of rectal GIST treated with Imatinib neoadjuvant therapy followed by trans anal local resection.
Key words. Gastrointestinal stromal tumours, rectal neoplasms, case reports, neoadjuvant therapy.
Resección del GIST rectal tras la terapia neoadyuvante. Relato de dos casos de un único instituto
Resumen
Los tumores del estroma gastrointestinal (GIST) son las neoplasias más comunes que se originan en las células mesenquimales del tracto gastrointestinal. Aunque los GIST pueden surgir en cualquier sitio gastrointestinal, los más frecuentes son el estómago y el intestino delgado. En el recto se localizan menos del 5% de todos los GIST. La cirugía es la principal opción de tratamiento para los GIST resecables. Actualmente, la escisión local se emplea cada vez más gracias a la terapia neoadyuvante. Este artículo describe dos casos de GIST rectal tratados con terapia neoadyuvante Imatinib seguida de resección local transanal.
Palabras claves. Tumores del estroma gastrointestinal, neo-plasias del recto, informes de casos, terapia neoadyuvante.
Introduction
Gastrointestinal stromal tumours (GIST) are the most common neoplasms arising from the mesenchymal cells (mesoderm) of the gastrointestinal tract.1 The development of this neoplasm is attributed to c-kit gene mutation or, less often, PDGFRA gene mutation.2 Although GIST can arise in any gastrointestinal site, the most common are stomach and small bowell.3 Rectal GIST accounts for less than 5% of all GIST.4
Surgery is the main treatment option for resectable GIST. However, even after a curative resection, recurrence and progression rates are around 40-60% after 2 years.5
Concerning rectal GIST, previous treatment were broad resections, such as pelvic exenteration and abdominoperineal rectal amputation.6, 7 Currently, thanks to neoadjuvant therapy, local excision has been increasingly employed.8
GIST usually responds to tyrosine kinase inhibitors, such as Imatinib and Sunitinib. These medications can be used as neoadjuvant therapy, reducing tumour size and increasing resect ability. Also, tyrosine kinase inhibitors potentially could increase the probability of sphincter preservation and reduce the need for rectal amputation.9
This study address two cases of rectal GIST treated by Imatinib neoadjuvant therapy followed by trans-anal local resection. These were two consecutive cases managed in a single institute. This study is in accordance with the declaration of Helsinki.
Presentation of cases
Case 1
A 56-years-old male patient was complaining of tenesmus and perianal pain for over a year. Rectal digital exam revealed a 7 cm mass in the left rectal wall, right above the dentate line.
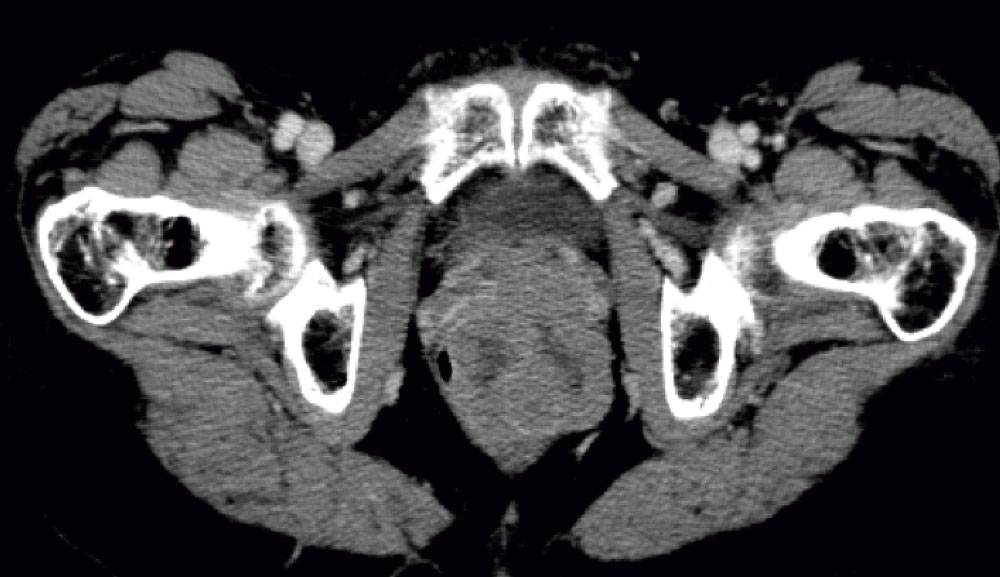
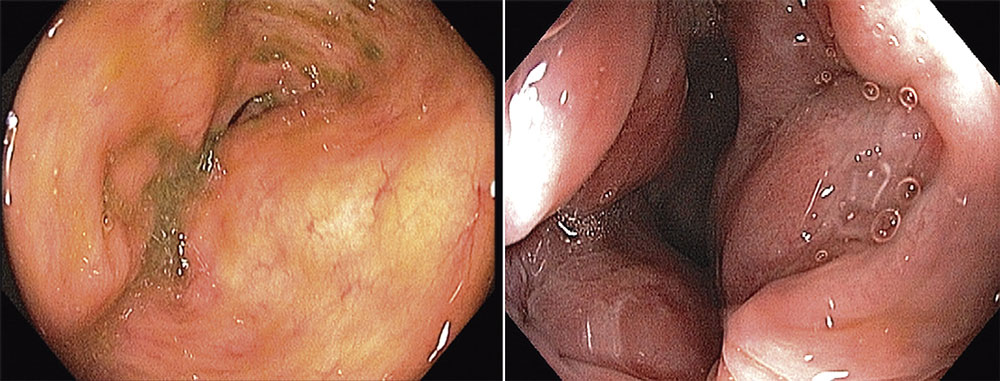
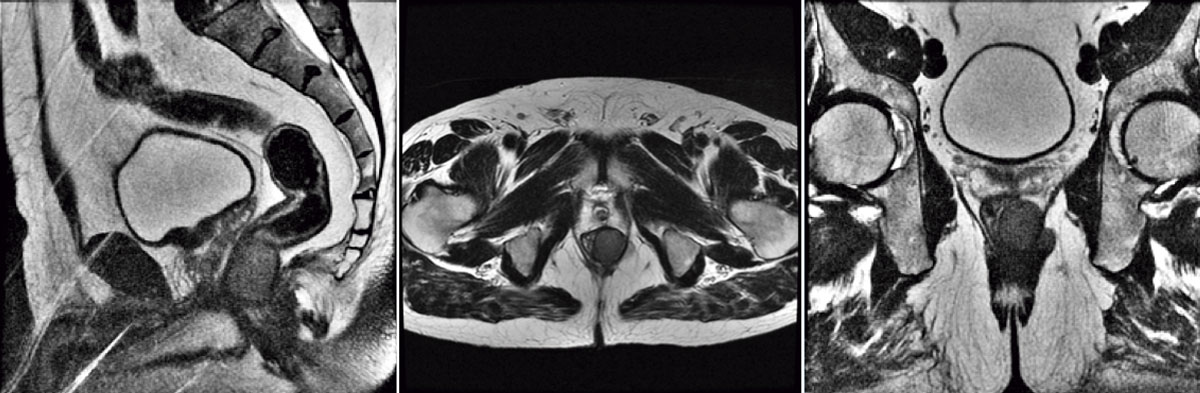
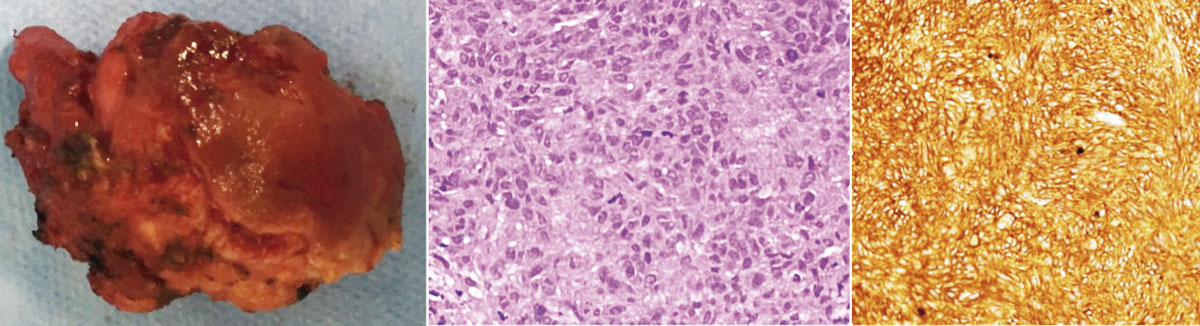
Colonoscopy evinced a round and regular mass, covered by normal mucosa. A trans-rectal biopsy was performed. Histological analysis showed epithelioid neoplasm with moderate atypia, certain necrotic areas, and up to 3 mitosis per 10 high-power field (HPF). Immunohistochemically positivity for CD117 (c-kit) and negative for smooth muscle actin or S-100 protein confirmed the diagnosis of a submucosal rectal GIST (Figure 1). Magnetic resonance evidenced a lesion invading external anal sphincter and reaching the mesorectal fat (Figure 2).
Patient received neoadjuvant therapy with Imatinib (400 mg per day) during 6 months.
After that, patient was submitted to trans-anal resection. The postoperative period was uneventful. The length of hospital stay was 4 days.
Histological analysis showed residual GIST measuring 4.3 cm, with viable cells comprising 5% of tumoral mass (Figure 3). Margins were negative.
Patient was submitted to adjuvant Imatinib. Following 2 years follow-up, the patient did not show any sign of recurrence and had no intestinal complaints.
Case 2
A 63-year-old female patient was complaining of constipation. Rectal digital exam revealed a 5 cm mass in the left anterior rectal wall, located 2 cm above the dentate line. Colonoscopy showed a round mass with normal mucosa. A biopsy was performed and immunohistochemically positivity for CD117 and CD34 antigens confirmed the diagnosis of a submucosal rectal GIST. The mitotic activity was 2×10 HPF, and no necrosis was identified.
Computed tomography showed the presence of a tumour in contact with the vaginal wall and invasion of the levator ani muscle (Figure 4).
Neoadjuvant therapy with Imatinib (400 mg per day) was started for a period 6 months long, and was interrupted due to myalgia.
The patient was then treated by local excision using the trans-anal endoscopic microsurgery technique. The tumour mass was completely excised with part of the posterior wall of the vagina and a minimal portion of tissue from the levator ani muscle.
The rectal and vaginal walls defect was closed primarily. A laparoscopic loop ileostomy was established. The postoperative period was uneventful and patient was discharged on 4th postoperative day. The specimen margins were negative. The lesion expressed CD34 or CD117 and showed no mitotic activity (zero mitoses in 50 HPF). Patient received adjuvant therapy with Imatinib for 3 years.
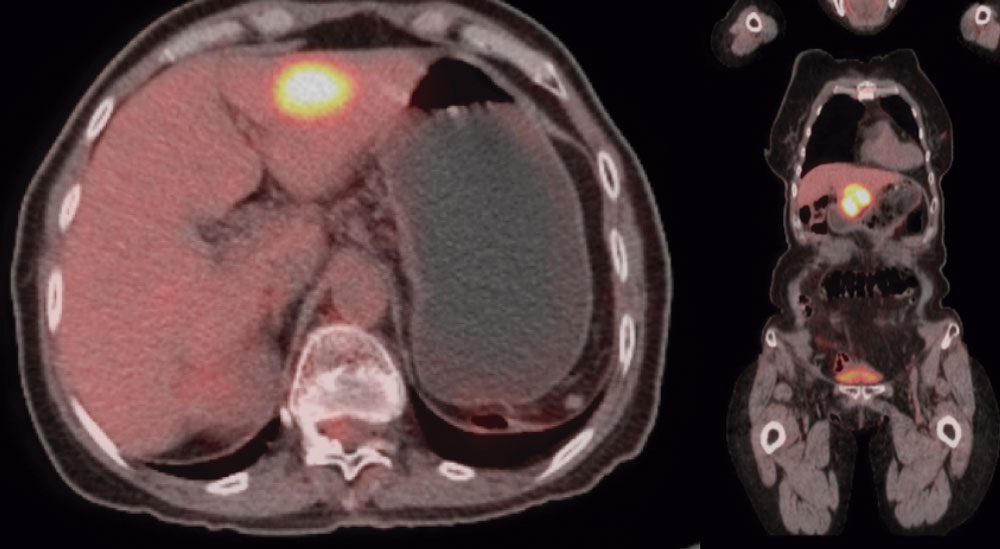
Four years after the surgery, patient underwent a new cycle of Imatinib and subsequent liver surgery, due to a liver nodule (segment III) detected with a PET-Scan exam. (Figure 5).
Intraoperative ultrasound identified two other nodules (liver segments V and VI). Patient underwent liver segmentectomy (segment III); nodulectomy (nodule in segment VI); radiofrequency ablation (nodule in segment). The postoperative period was uneventful, and patient was discharged on the 6th postoperative day. Immunohistochemistry was positive for c-kit and CD34 for both resected nodules.
Once recurrence developed after Imatinib was stopped, chronic Imatinib therapy was indicated. After 6 years after liver surgery, the patient is now disease-free and has no complaints, still under Imatinib therapy, and no signs of toxicity.
Discussion
The surgical approach is the main step for curative intent GIST management. The surgical procedure aims primarily to remove the tumour with R0 resection, as well as its surrounding pseudo capsule, without rupturing it, and avoiding tumoral bleeding.14
Neoadjuvant therapy prior to resection may be considered in cases where poor prognostic factors, such as big tumour size or high mitotic index are identified. Also, neoadjuvant therapy should be considered in cases of neoplasms next to important structures, such as great vessels or anal sphincter.15
Liu et al.9 reported 21 cases of rectal GIST resection, of which 13 underwent local excision (10 trans-anal, 2 Tran sacral, 1 transvaginal), and 8 patients underwent transabdominal approach. Trans anal approach was used for most small tumours of distal rectum. Tran-sacral approach was used for medium rectum. Neoadjuvant Imatinib was used in 5 patients, and those who received preoperative Imatinib therapy had a higher rate of negative resection margins than those without preoperative Imatinib therapy.
Adjuvant therapy should be considered in patients with significant risk of recurrence after GIST resection. Patient risk of recurrence is based upon tumour location, size and mitotic count.16, 17 The SSG XVIII trial,18 a large, Scandinavian, prospective, randomized phase 3 trial, demonstrated superior relapse-free survival with 3 years of adjuvant IM compared with 1 year. Both of the current cases underwent Imatinib adjuvant therapy. The second patient developed recurrence after Imatinib stopped, and thus, a chronic therapy (> 5 years) was indicated after metastases resection.
Although genetic testing was not performed in the present study, mutation status of the primary tumour or metastasis could also be considered in assessing patient prognosis. It is well known that certain mutations may predict Imatinib response.18 As tumour sequencing becomes faster and more widespread, neoadjuvant, adjuvant and palliative therapies will become more tailored.
Conclusion
The current study report two cases of rectal GIST treated with neoadjuvant therapy, which allowed local excision, preserving sphincter and with a fast and satisfactory postoperative recovery.
Up to the current manuscript publication, both cases are disease-free.
Conflict of interest. Authors have no conflict of interest. The Institutional Ethics Committee approved this study protocol.
Financial support. This study did not have any sources of funding.
References
- Ducimetière F, Lurkin A, Ranchère-Vince D, Decouvelaere AV, Péoc’h M, Istier L, Chalabreysse P, Muller C, Alberti L, Bringuier PP, Scoazec JY, Schott AM, Bergeron C, Cellier D, Blay JY, Ray-Coquard I. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One 2011; 6 (8): e20294.
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998; 279 (5350): 577-580.
- Joensuu H, DeMatteo RP. The management of gastrointestinal stromal tumours: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med 2012; 63: 247-258.
- Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumours: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era-a population-based study in western Sweden. Cancer 2005; 103 (4): 821-829.
- Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumours: before and after STI-571. Hum Pathol 2002; 33 (5): 466-477.
- Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR. Tumour mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumour (GIST). Cancer 2008; 112 (3): 608-615.
- Miettinen M, Lasota J. Gastrointestinal stromal tumours: pathology and prognosis at different sites. Semin Diagn Pathol 2006; 23: 70-83.
- Cavnar MJ, Wang L, Balachandran VP, Antonescu CR, Tap WD, Keohan M, Singer S, Temple L, Nash GM, Weiser MR, Guillem JG, Aguilar JG, DeMatteo RP, Paty PB. Rectal Gastrointestinal Stromal Tumour (GIST) in the Era of Imatinib: Organ Preservation and Improved Oncologic Outcome. Ann Surg Oncol 2017; 24 (13): 3972-3980. doi: 10.1245/s10434-017-6087-9.
- Liu H, Yan Z, Liao G, Yin H. Treatment strategy of rectal gastrointestinal stromal tumour (GIST). J Surg Oncol 2014; 109: 708-713.
- Tielen R, Verhoef C, van Coevorden F, Reyners AK, van der Graaf WT, Bonenkamp JJ, van Etten B, de Wilt JH. Surgical management of rectal gastrointestinal stromal tumors. J Surg Oncol 2013; 107 (4): 320-323.
- Jakob J, Mussi C, Ronellenfitsch U, Wardelmann E, Negri T, Gronchi A, Hohenberger P. Gastrointestinal stromal tumour of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol 2013; 20 (2): 586-592.
- Stiekema J, Kol S, Cats A, Yazdi AT, van Coevorden F, van Sandick JW. Surgical Treatment of Gastrointestinal Stromal Tumours Located in the Stomach in the Imatinib Era. Am J Clin Oncol 2015; 38 (5): 502-507.
- Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, Fletcher CD, Demetri GD, Bertagnolli MM. Surgical management of advanced gastrointestinal stromal tumours after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006; 24 (15): 2325-2331.
- Chaudhry UI, DeMatteo RP. Advances in the surgical management of gastrointestinal stromal tumour. Adv Surg 2011; 45: 197-209.
- Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumour. N Engl J Med 2001; 344 (14): 1052-1056.
- Reichardt P, Blay JY, Boukovinas I, Brodowicz T, Broto JM, Casali PG, Decatris M, Eriksson M, Gelderblom H, Kosmidis P, Le Cesne A, Pousa AL, Schlemmer M, Verweij J, Joensuu H. Adjuvant therapy in primary GIST: state-of-the-art. Ann Oncol 2012; 23 (11): 2776-2781.
- Etherington MS, DeMatteo RP. Tailored management of primary gastrointestinal stromal tumours. Cancer 2019.
- Joensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hartmann JT, Pink D, Ramadori G, Hohenberger P, Al-Batran SE, Schlemmer M, Bauer S, Wardelmann E, Nilsson B, Sihto H, Bono P, Kallio R, Junnila J, Alvegård T, Reichardt P. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol 2016; 34 (3): 244-250.
Correspondencia: Francisco Tustumi
Av Dr. Eneas de Carvalho 255 (Code: 05403-000). Cerqueira Cesar, San Pablo, Brasil
Tel.: +55 11 – 998791048 / Fax: +55 18-35812008
Correo electrónico: franciscotustumi@gmail.com
Acta Gastroenterol Latinoam 2020;50(2):154-158
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE