Lucas Souto Nacif, Rubens Macedo Arantes, Rodrigo Bronze Martino, Wellington Andraus, Luiz Carneiro D’ Albuquerque
Liver and Gastrointestinal Transplant Division. Department of Gastroenterology, University of São Paulo School of Medicine. São Paulo, Brazil.
Acta Gastroenterol Latinoam 2016;46: 383-385
Recibido: 08/12/2015 / Aprobado: 22/08/2016 / Publicado en www.actagastro.org el 01/01/2017
Summary
Pancreatic cystic lesions with increasing advances imaging and asymptomatic cystic tumors of the pancreas has increased than are described prevalence in the literature between 2.6-19.6%. These neoplasms are almost exclusively benign tumors, and rarely become malignant. The aim of this paper was to review the literature related to pancreatic serous cystic. Method. Non-Systematic literature review through Medline-PubMed database with headings related to pancreas serous cystadenoma in articles published until June 2015 was done. Discussion. Overall, symptomatic serous cystic neoplasms should always be resected. The presence of symptoms and/or the inability to definitively exclude a premalignant or malignant tumor are considered indications for surgical resection, and the tumor size at presentation is not essential when making the decision to perform surgery. Conclusion. Unless the diagnosis of serous cystadenoma is certain, cystic tumors with inconclusive clinical and imaging features should be radically treated.
Key words. Cancer of pancreas, serous cystadenoma, surgical procedures, operative.
Cistoadenoma seroso pancreático: diagnóstico y manejo terapéutico
Resumen
De las lesiones quísticas pancreáticas, con el avance de las imágenes, los tumores quísticos serosos asintomáticos han aumentado su prevalencia y están descritas en la literatura entre el 2,6 a 19,6%. Estos tumores son casi exclusivamente benignos y rara vez se convierten en malignos. El objetivo de este estudio fue revisar la literatura relacionada con las lesiones quísticas serosas del páncreas. Método. Se realizó una revisión no sistemática de la literatura utilizando Medline, base de datos de PubMed, con los términos y descriptores relacionados de cistoadenoma seroso del páncreas en los artículos publicados hasta junio de 2015. Discusión. En general, los tumores quísticos serosos sintomáticos siempre deben ser resecados. La presencia de síntomas y/o la incapacidad para descartar permanentemente una lesión pre maligna o maligna se consideran indicaciones para la resección quirúrgica. El tamaño del tumor en el momento de la presentación no es esencial para la toma de decisión de realizar una cirugía. Conclusión. A menos que el diagnóstico de cistoadenoma seroso sea muy sugestivo, los tumores quísticos con características clínicas y de imagen no concluyentes deben ser tratados de manera radical.
Palabras claves. Cáncer de páncreas, cistoadenoma seroso, procedimientos quirúrgicos.
Cystic tumors of the pancreas prevalence found in the literature are estimated between 2.6-19.6% and its frequency is increasing due to the advances in imaging for diagnosis and asymptomatic cystic tumors of the pancreas has increased.1 Clarification of the clinical, pathological and imaging findings of serous cystic neoplasm of the pancreas is needed, and the indications for surgery in patients with these neoplasms are occasionally controversial.2, 3
Serous cystic neoplasms are almost exclusively benign tumors, even in their different morphological presentations (microcystic, oligocystic/macrocystic) and cystadenocarcinoma, which is infrequently reported.1-4 Thus, 40-75% of patients with cystic pancreatic tumors are asymptomatic,4 and the other cases are incidentally detected by imaging analysis.3, 6 The aim of this paper was to review the literature related to pancreatic serous cystic.
Method
Non-Systematic literature review through Medline- PubMed database with headings related to pancreas serous cystadenoma in articles published until June 2015 was done.
Discussion
Serous microcystic adenomas are benign tumors of the pancreas and constitute approximately 1-2% of all exocrine pancreatic tumors.6, 7 Almost all serous microcystic adenomas are benign, and they rarely progress to malignancy.5 The differential diagnosis for this disease includes mucinous cystic neoplasms, lymphangiomas, acinar cell cystadenocarcinomas, and serous cystadenocarcinomas.5 The most important differential diagnosis is the distinction between mucinous and non-mucinous cystic lesions. A meta analysis demonstrated that EUS with cyst fluid analysis could differentiate between mucinous and non-mucinous lesions with a sensitivity of 63% and specificity of 88%.8
Serous microcystic adenomas commonly present in older women, in the seventh to eighth decades of life. They have a female predominance of 70%.6, 7 Two thirds of patients present with symptoms, such as abdominal pain, an abdominal mass, nausea, vomiting, or weight loss. The remaining one third of tumors are incidentally discovered by routine physical and imaging examination or at autopsy.6, 7 Overall, symptomatic tumors are more expected to be malignant.
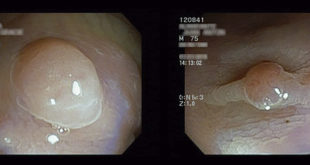
Imaging diagnosis of serous cystic neoplasm is not simple. Tumors of the pancreas that cannot be confirmed to be serous cystic neoplasms, which have the potential to become malignant, should be resected because of the possibility of pancreatic cancer.2 Most of the serous cystic neoplasms are developed in the tail or body of the pancreas; however, they have also been described in the head of the pancreas. Their size ranges from 1 to 25 cm, with an average size of 6 to 10 cm.6, 7
Usually, the diagnosis is performed using percutaneous or endoscopic ultrasonography (EUS) with guided aspiration or puncture and analysis of cystic fluid content, including amylase activity, viscosity, mucin presence, cytology and tumor marker levels.4 EUS with cyst fluid analysis was examined in a meta-analysis and was shown to differentiate between mucinous and non-mucinous lesions with a high sensitivity and a specificity.8 Eight microcystic serous cystadenomas with subtotal cystic degeneration were retrieved from among 397 resected serous cystadenomas (2.0%). Available radiographic studies showed classic features findings in 2 of 4 cases. Four cysts were unilocular, and 4 were multilocular.12 In clinical practice, cystic fluid analysis should be interpreted in combination with CT/MRI imaging and EUS to help in the diagnosis of unclear cases.7
Histologically, serous cystic neoplasms are considered to originate from the ductal epithelium of the pancreas. They appear as epithelial cells connected by occluding junctions and belt desmosomes resting on a basement membrane and have a central scar. Immunohistochemically, they are positive for cytokeratins 7, 8, 18, and 19. They can also be positive for CA19-9 and B72.3, being negative for CEA.5
Overall, symptomatic serous cystic neoplasms should always be resected.7 The presence of symptoms and/or the inability to definitively exclude a premalignant or malignant tumor (oligo and/or macrocystic lesions) are considered indications for surgical resection, and the tumor size at presentation is not essential when making the decision to perform surgery.7 A fair consensus is that lesions that are asymptomatic and/or < 4 cm in size can be followed up at early intervals, while surgery should be offered to patients with symptomatic lesions and tumors > 4 cm, because they have been seen to grow at a rate of almost 1.98 cm/year.11
A multinational study of 2.622 patients, showed 74% were women, and median age at diagnosis was 58 years (16-99). Patients presented with non-specific abdominal pain (27%), 52% of patients were operated on during the first year after diagnosis, median size: 40 mm (range: 2-200), 9% had resection beyond 1 year of follow-up: 3 years (range: 1–20), size at diagnosis: 25 mm (range: 4-140) and 39% had no surgery 3.6 years (range: 1–23), 25.5 mm (range: 1–200). Surgical indications were uncertain diagnosis (60%), symptoms (23%), size increase (12%), large size (6%) and adjacent organ compression (5%). In patients followed beyond 1 year (n = 1271), size increased in 37% (growth rate: 4 mm/year), was stable in 57% and decreased in 6%. Three serous cystadenocarcinomas were recorded. Postoperative mortality was 0.6% (n = 10), and serous cystic neoplasm related mortality was 0.1% (n = 1).1
In a recent series, so-called locally aggressive behavior, defined as invasion of surrounding vessels or peripancreatic lymph nodes, was described in 5.1% of resected serous cystic neoplasms. Large tumor size (> 6 cm) and tumor location in the head of the pancreas were considered independent risk factors for this aggressive behavior and to possibly justify surgical resection.7-10
In summary, unless the diagnosis of serous cystadenoma is certain, cystic tumors with inconclusive clinical and imaging features should be radically treated.
Acknowledgements. We thank Dr Agustin Vintimilla who provided medical writing services on behalf of Department of Gastroenterology.
Supportive foundation. The authors declare that they don’t have any support.
Conflict of interest. The authors declare that they have no conflict of interest with regards to the content of this manuscript.
Referencias
- Jais B,Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, Bassi C, Manfredi R, Moran R, Lennon AM, Zaheer A, Wolfgang C, Hruban R,Marchegiani G, Fernández Del Castillo C, Brugge W, Ha Y, Kim MH, Oh D, Hirai I, Kimura W, Jang JY, Kim SW, Jung W, Kang H, Song SY, Kang CM, Lee WJ, Crippa S, Falconi M, Gomatos I, Neoptolemos J, Milanetto AC, Sperti C, Ricci C, Casadei R, Bissolati M, Balzano G, Frigerio I, Girelli R, Delhaye M, Bernier B, Wang H, Jang KT, Song DH, Huggett MT, Oppong KW, Pererva L, Kopchak KV, Del Chiaro M, Segersvard R, Lee LS, Conwell D, Osvaldt A, Campos V, AgueroGarcete G, Napoleon B, Matsumoto I, Shinzeki M, Bolado F, Fernandez JM, Keane MG, Pereira SP, Acuna IA, Vaquero EC, Angiolini MR, Zerbi A, Tang J, Leong RW, Faccinetto A, Morana G, Petrone MC,Arcidiacono PG, Moon JH, Choi HJ, Gill RS , Pavey D, Ouaïssi M, Sastre B, Spandre M, De Angelis CG, Rios-VivesMA, Concepcion-Martin M, IkeuraT,Okazaki K,Frulloni L, Messina O, Lévy P.Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut. 2016; 65: 305-312.
- Hashimoto M, Watanabe G, Matsuda M, Mori M. Serous cystic neoplasm of the pancreas-indications for surgery. Hepatogastroenterology 2006; 53: 950-952.
- Hutchins GF, Draganov PV. Cystic neoplasms of the pancreas: a diagnostic challenge. World J Gastroenterol 2009; 15: 48-54.
- Sarr MG, Kendrick ML, Nagorney DM, Thompson GB, Farley DR, Farnell MB. Cystic neoplasms of the pancreas: benign to malignant epithelial neoplasms. SurgClin North Am 2001; 81: 497-509.
- Omeroglu A, Paner GP, Ciesla MC, Hartman G. Serous microcystic adenoma of the pancreas. Arch Pathol Lab Med 2001; 125: 1613-1644.
- Ip IK, Mortele KJ, Prevedello LM, Khorasani R. Focal cystic pancreatic lesions: assessing variation in radiologists management recommendations. Radiology 2011; 259: 136-141.
- Del Chiaro M, Verbeke C, Salvia R, Klöppel G, Werner J, McKay C, Friess H, Manfredi R, Van Cutsem E, Löhr M, Segersvärd R; European Study Group on Cystic Tumours of the Pancreas. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 2013; 45: 703-711.
- Thosani N, Thosani S, Qiao W, Fleming JB, Bhutani MS, Guha S. Role of EUS-FNA-based cytology in the diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Digestive Diseases and Sciences 2010; 55: 2756-2766.
- Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms of the pancreas revisited. Part IV: Rare cystic neoplasms. Surg Oncol 2012; 21: 153-163.
- Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multi institutional retrospective study of 398 cases. French Surgical Association. Ann Surg1999; 230: 152-161.
- Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez del Castillo C. Serouscystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg 2005; 242: 413-419.
- Panarelli NC, Park KJ, Hruban RH, Klimstra DS. Microcystic serous cystadenoma of the pancreas with subtotal cystic degeneration: another neoplastic mimic of pancreatic pseudocyst. Am J Surg Pathol 2012; 36: 726-731.
Correspondencia: Lucas Souto Nacif
Tel.: +55-11-26613323 Fax: +55-11-26619008
Correo electrónico: lucasnacif@usp.br
Acta Gastroenterol Latinoam 2016;46(4): 383-385
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE