Carolin Desire Nava, Carlos David Porón, José Celso Ardengh
Endoscopic Service of the 9 de Julho Hospital Sao Paulo, Brazil.
Acta Gastroenterol Latinoam 2017;47(2):144-147
Recibido: 12/12/2016 / Aprobado: 24/02/2017 / Publicado en www.actagastro.org el 03/07/2017
Summary
Introduction. Acute pancreatitis is an inflammation of the pancreas that can cause systemic inflammatory response syndrome and develop therefore according to the disease’s severity a significant rate of morbidity and mortality. Cholelithiasis and alcohol consumption are the most frequent causes. The occurrence of recurrent episodes of acute pancreatitis caused by pancreatic cancer is rare. The authors present a case of recurrent acute pancreatitis caused by a rare pancreatic neoplasia. Case report. A 35-year-old female patient presented abdominal pain of high intensity in the upper right quadrant with 15 days of evolution, also associated with nausea and vomiting. After the onset of pain, jaundice and colure appeared gradually, without clinical improvement, because of this, the patient decides to come to our emergency service. At admission, amilase was elevated five times the upper limit of normal; gamma-glutamyltransferase, aspartate aminotransferase and alanine aminotransferase (GGT, TGP and TGO), were also high. Elevated bilirubin at the expense of the direct fraction were observed too. He reports that 6 years previously, he presented two cases of extrahepatic cholestasis, the first one being associated with acute pancreatitis. The patients also refer another two episodes of acute pancreatitis.
Key words. Acute pancreatitis, EUS staging, pancreatic neoplasms, acinar cell carcinoma, diagnosis, treatment.
Una causa poco frecuente de pancreatitis aguda recurrente
Resumen
Introducción. La pancreatitis aguda es una inflamación del páncreas que puede conllevar a un síndrome de respuesta inflamatoria sistémica y con esto desencadenar diversas consecuencias de morbilidad y mortalidad según la severidad de la misma. En la actualidad su incidencia es cada vez mayor, representando una de las causas más comunes de hospitalización por causa gastrointestinal. Litiasis vesicular y el consumo de alcohol son las causas más frecuentes. La pancreatitis aguda recurrente, sigue siendo un desafío a nivel clínico y terapéutico, especialmente por sus múltiples causas y su presentación debido a cáncer de páncreas es extremadamente rara. Presentamos un caso de pancreatitis aguda recurrente causada por una neoplasia de páncreas. Caso clínico. Paciente femenina de 35 años con dolor abdominal de moderada a fuerte intensidad en hipocondrio derecho sin irradiación que cede con la administración de antiespasmódico, concomitantemente náuseas y vómitos de 15 días de evolución, posteriormente se asocia al cuadro clínico ictericia, coluria sin mejoría clínica, motivo por los cuales acude a nuestro centro. Al ingreso presenta amilasa elevada 5 veces el límite normal. y glutamil transpeptidasa, aspartato y alanino transferasa (GGT, TGP y TGO) están elevadas. Bilirrubinas a predominio de la fracción directa. Como antecedente refiere 6 años dos cuadros de colestasis extra hepática, siendo el primero de ellos asociado a pancreatitis aguda. Así mismo otros dos episodios más de pancreatitis aguda.
Palabras claves. EUS estatificación, neoplasias pancreáticas, carcinoma de células acinares, diagnóstico, tratamiento.
Case report
35-year-old female patient presented for two weeks high-intensity abdominal pain predominantly located in the right upper quadrant, it is associated with nausea and vomiting. After the onset of pain, jaundice and dark urine appeared. Amylase greater than 894 U/L, lipase = 2653 U/L, AST = 1345 U/L, ALT = 1876 U/L, and GGT = 678 U/L. Bilirubin was high with a greater direct fraction. Transabdominal ultrasound (US) and magnetic resonance imaging (MRI) were performed (Figure 1). Six years before she presented two episodes of extrahepatic cholestasis, the first one was associated with acute pancreatitis. The second episode was associated with jaundice and was performed endoscopic retrograde cholangiopancreatography (ERCP) that find common bile duct stones and dilated bile duct showed by US. Papillotomy and mechanical biliary clearance were performed. She had undergone a cholecystectomy one week after these symptoms. Two years after she presented a new pain episode, associated with AST and ALT elevation more than 20 times the normal and serum amylase more than 3 times normal. The treatment was conventional. The evolution was good until this new episode of acute pancreatitis. The patient was submitted to MRI (Figure 1) and a new ERCP (Figure 2). Because of this the patient was carried to EUS with guided fine needle aspiration biopsy (EUS-FNA) who found hyper vascular lesion in pancreatic isthmus. The diagnosis of EUS microhistology was acinar cell carcinoma (Figure 3). Patient undergoing to gastroduodenopancreatectomy. Tumor pathology report a 4.0 x 3.0 x 3.0 cm mass (Figure 4). Well-differentiated acinar cells carcinoma unifocal, solid and cystic, with mitotic activity 2/10. No evidence of vascular, lymphatic and perineural invasion. Some foci of necrosis present. Chronic pancreatitis changes were found in the underlying pancreatic parenchyma. Disease-free margins were obtained. Immunohistology was positive for chymotrypsin and trypsin (Figures 5 and 6) Pathological stage: pT2pN0 (tumor larger than 2 cm, restricted to the pancreas). After 8 years’ follow-up, the patient was in good health condition.
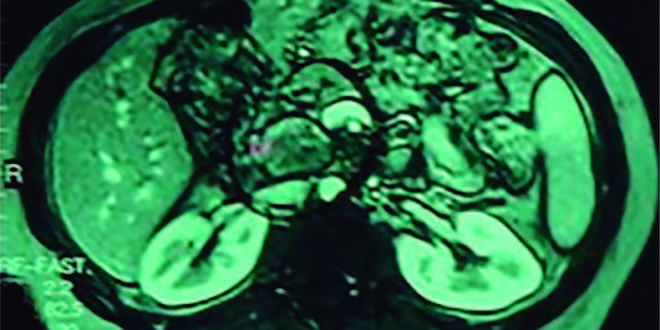
Figure 1. MRI of the upper abdomen shows a solid-cystic pancreatic lesion well circumscribed of 3 cm located in the head of the pancreas with a dilation of the common bile duct and the hypodense image inside the main pancreatic duct with the dilation.

Figure 2. The same findings were observed in the ERCP.
Figure 3. EUS imaging showed a hypoechoic mass (tumor) inside the main pancreatic duct (yellow arrows).
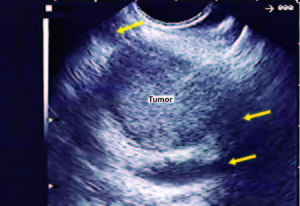
Figure 4. Product of the GDP (surgical specimen).
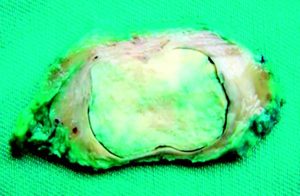
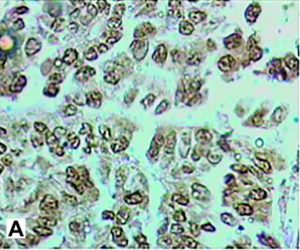
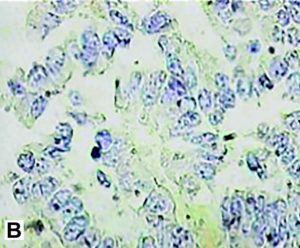
Figure 5. Immunohistology was positive for chymotrypsin(A) and trypsin (B).
Discussion
Acinar cell carcinoma (ACC) is an epithelial tumor of pancreas, considered as a rare neoplasia and accounting 1-2% of exocrine pancreatic neoplasms, defined by its resemblance to the acinar cells and exocrine pancreatic enzyme production. The acinar differentiation is defined as production of zymogen granules, most of them are solid and malignant, only acinar cells cystadenoma are benign, the rest are acinar cystoadenocarcinoma and mixed variants with neuroendocrine and ductal carcinoma. To define as acinar must not exceed 25% of endocrine or ductal cells.1 Is more common in the pancreatic head, men ratio 3.6:1 compared with women, with a peak incidence in the sixth decade,1, 2 but approximately 6% ACCs occur in childhood, and 15% of pediatric pancreatic neoplasms are ACCs.2, 3
This tumor does not have typical presentations, because is relative circumscribed, invasion of the biliary system is less frequent than another carcinomas;3 conventionally the symptoms are related to mass effect. These include weight loss, abdominal pain, vomiting and general weakness. Ten and fifteen percent of patients have lipase hypersecretion syndrome, presenting skin nodules related with subcutaneous fat necrosis and polyarthralgia related with secondary cancellous bone sclerotic lesions; the syndrome is associated with liver metastases.1 Although 13.8% ductal adenocarcinoma patient presents as acute pancreatitis, this is exceptional in ACC. Thomas et al, reported a case of a 70 years-old male patient with acute pancreatitis initial presentation.4 Fabre et al, reported a case of intraductal ACC who presented with recurrent acute pancreatitis, but was associated with neuroendocrine microadenoma.5 This case is a young patient with three episodes of acute pancreatitis and two of cholestasis in 5-years. At diagnosis, the average tumor size is 10 cm, usually are round, with well-defined contour, a homogeneous contrast uptake, but lower than the normal surrounding parenchyma. No specific laboratory abnormalities, but some patients have elevated serum lipase. EUS-FNA cytology is usually required for histological diagnosis often is obtained acinar cell differentiation sample, cell cords and solid nests of a neoplastic epithelium1 that happened to our patient. Grapes bunch pattern is not common. Some studies has been trying to relate the utility of EUS elastography and contrast-enhanced EUS for advantage in the differential diagnosis of acinar cell carcinoma from pancreatic ductal adenocarcinoma without success.6 The diagnostic hallmark of ACC is the immunohistochemically demonstration of acinar-specific products such as trypsin, lipase, amylase, and carboxyl ester lipase (CEL).7 Klimstra and Sigel found differences between cytology and histologic diagnosis in 14 of 29 cases.8 Acinar carcinoma cytology was misdiagnosed as a neuroendocrine tumor (NET) or ductal adenocarcinoma in 13 cases.7 ACC granular chromatin without uniform distribution pattern differs with salt and pepper NET pattern. Immunohistologically chymotrypsin and trypsin are positive in ACC, but often negative in NET and solid pseudo papillary tumors (SPT). Five percent of NET shows positive enzyme markers. B-catenin is positive in nucleus and cell membrane ACC, while NET only present in the membrane. The strong expression of vimentin can help differentiate pseudo papillary solid tumor and ACC.9 Macroscopically are usually large, well circumscribed and multinodular. Grouped nests pyramidal cells are observed in a small lumen at microscopic analysis, with pure solid or acinar pattern.1 The prognosis is better compared PDAC but still is a highly aggressive neoplasm. The median survival for localized and metastatic disease is 38 and 14 months, and the overall 5-years survival is less than 10%. Fifty percent of patients have metastases at diagnosis, and 50% develop additional metastases after surgical resection. Metastasis is more common to regional lymph nodes and liver. Factors associated with poor prognosis are age over 60 years, male gender and tumor larger than 10 cm. The classification stage is the same in all exocrine pancreatic carcinoma cells. The only potentially curative therapy is surgical excision with margins and lymph nodes free of disease, and the absence of metastasis.1 In the absence of data on prospective chemotherapeutic treatment regimens currently DAC or colorectal cancer are used. 5-FU is the agent used but described gemcitabine, cisplatin, doxorubicin and irinotecan among others. For locally advanced or metastatic disease, some studies speak in favor to the combination of oxaliplatin-based chemotherapy that appears to be most effective than others available treatments.10 Some reports show that in selected patients, repeated serum lipase measurements were accurately predicted response to chemotherapy and relapse after surgery.11 In unresectably disease, patients should be treated with 5-FU for neoadjuvant or palliative treatment.4 ACC of the pancreas is considered rare neoplasia, despite its poor overall survival, has a better prognosis than DAC.12 The diagnosis is often late and symptoms are unspecific, few reported initial presentation cases with acute pancreatitis are known.12 The pathological diagnosis is made with EUS-FNA since the differential diagnosis is wide. Immunohistochemically and enzymatic markers are necessary for an accurate cytological diagnosis. Surgery is the only chance of cure.
References
- Chaudhary P. Acinar Cell Carcinoma of the Pancreas: A Literature Review and Update. Indian J Surg 2015; 77: 226-231.
- Klimstra DS, Adsay V. Acinar neoplasms of the pancreas. A summary of 25 years of research. Klimstra DS, Adsay V. Semin Diagn Pathol 2016; 33: 307-318.
- Shorter NA, Glick RD, Klimstra DS, Brennan MF, Laquaglia MP: Malignant pancreatic tumors in childhood and adolescence: The Memorial Sloan-Kettering experience, 1967 to present. Journal of Pediatric Surgery 2002; 37: 887-892.
- Thomas PC, Nash GF, Aldridge MC. Pancreatic acinar cell carcinoma presenting as acute pancreatitis. HPB (Oxford) 2003; 5: 111-113.
- Fabre A, Sauvanet A, Flejou JF, Belghiti J, Palazzo L, Ruzniewski P, Degott C, Terris B. Intraductal acinar cell carcinoma of the pancreas. Virchows Arch 2001; 438: 312-315.
- Chantarojanasiri T, Hirooka Y, Kawashima H, Ohno E, Yamamura T, Funasaka K, Nakamura M, Miyahara R, Ishigami M, Watanabe O, Nakaguro M, Shimoyama Y, Nakamura S, GotoH. Endoscopic ultrasound in the diagnosis of acinar cell carcinoma of the pancreas: contrast-enhanced endoscopic ultrasound, endoscopic ultrasound elastography, and pathological correlation. Endosc Int Open 2016; 4: E1223-E1226.
- La Rosa S, Sessa F, Capella C. Acinar Cell Carcinoma of the Pancreas: Overview of clinicopathologic features and insights into the molecular pathology. Front Med (Lausanne) 2015; 2: 41.
- Sigel CS, Klimstra DS. Cytomorphologic and immunophenotypical features of acinar cell neoplasms of the pancreas. Cancer Cytopathol 2013; 121: 459-470.
- Yoneda M, Kanayama K, Imai H, Shiraishi T. Report of a case of acinar cell carcinoma with its differential diagnosis on endoscopic ultrasound-guided fine-needle aspiration cytology. J Cytol 2014; 31: 93-95.
- Béchade D, Desjardin M, Salmon E, Désolneux G, Bécouarn Y, Evrard S, Fonck M. Pancreatic acinar cell carcinoma. Case Rep Gastroenterol 2016; 10: 174-180.
- Kruger S, Haas M, Burger PJ, Ormanns S, Modest DP, Westphalen CB, Kleespies A, Angele MK, Hartwig W, Bruns CJ, Kirchner T, Werner J, Heinemann V, Boeck S. Acinar cell carcinoma of the pancreas: a rare disease with different diagnostic and therapeutic implications than ductal adenocarcinoma. J Cancer Res Clin Oncol 2016; 142: 2585-2591.
- Glazer ES, Neill KG, Frakes JM, Coppola D, Hodul PJ, Hoffe SE, Pimiento JM, Springett GM, Malafa MP. Systematic review and case series report of acinar cell carcinoma of the pancreas. Cancer Control 2016; 23: 446-454.
Correspondence: José Celso Ardengh
Alameda dos Arapanés, 881 –cj 111. CEP 04524-00, São Paulo, SP Brasil
Tel. (+55 11)996886312
Email: jcelso@uol.com.br
Acta Gastroenterol Latinoam 2017;47(2): 144-147
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE