Fernando D Saraví, Karina A González Otárula, Graciela E Carra, Jorge E Ibáñez
Instituto de Fisiología, Facultad de Ciencias Médicas, Universidad Nacional de Cuyo, Mendoza, Argentina.
Recibido: 10/03/2015 / Aprobado: 23/09/2015
Summary
Introduction. The colonic epithelium is a classical aldosterone target, but the effect of the hormone on the oxygen consumption rate (QO2 ) of this tissue is unknown. Objectives. We aimed at assessing, in the rectal epithelium of rats fed with diets of different sodium content, the effect of epithelial sodium channel (ENaC) blockade on short-circuit current (ISC ) and QO2 , and the acute effect of aldosterone incubation on ISC and QO2 . Methods. Adult male rats were fed with normal, low or high-sodium diets for 8 days. Plasma sodium and serum aldosterone were measured. Isolated mucosa preparations from the rectal portion of the colon were mounted in Ussing chambers modified to measure ISC and QO2. Results. Baseline ISC and QO2 were highest in sodium-deprived rats. Both were proportionally reduced by amiloride (0.1 mM) in this group and in the normal sodium group, but not in sodium-loaded rats. In separate experiments, incubation with aldosterone (10 nM) for 7 h increased ISC and QO2 in all groups; increases were larger in the normal and sodium-loaded groups. Amiloride decreased both ISC and QO2 , abolishing the differences between groups. Linear regression of the decrease in QO2 and ISC after amiloride showed the steepest slope for the sodium-deprived group and the flattest one for the sodium-loaded group. Conclusions. Baseline epithelial QO2 of sodium-deprived and control rats is reduced by ENaC blockade. Aldosterone increased QO2 proportionally to ISC augmentation in all groups, but the coupling between aerobic metabolism and electrogenic transport seems more efficient in sodium-deprived animals.
Key words. Aldosterone, ENaC, oxygen consumption, rat rectal colon, short-circuit current, sodium intake.
La aldosterona aumenta el consumo de oxígeno del epitelio rectal de ratas normales, privadas de sodio y cargadas de sodio
Resumen
Introducción. El colon es un blanco clásico de la aldosterona, pero su efecto sobre el consumo de oxígeno (QO2) del epitelio se desconoce. Objetivos. Evaluar el efecto del bloqueo de canales epiteliales de sodio (ENaC) sobre la corriente de cortocircuito (ISC ) y el QO2 del epitelio rectal de ratas con diferentes ingestas de sodio, y el efecto agudo de la incubación con aldosterona sobre ISC y QO2. Métodos. Ratas machos adultas recibieron dietas de contenido bajo, normal o elevado de sodio por 8 días. Se midió sodio plasmático y aldosterona sérica. Se montaron preparaciones de mucosa aislada en una cámara de Ussing modificada para medir ISC y QO2 . Resultados. Los valores basales de ISC y QO2 fueron máximos en las ratas privadas de sodio. En éstas y las normales, pero no en las cargadas con sodio, amilorida (0,1 mmol/L) redujo ISC y QO2. En experimentos separados, la incubación con aldosterona (10 nmol/L) por 7 h aumentó ISC y QO2 en los tres grupos; los aumentos fueron mayores en las ratas normales y cargadas de sodio. La amilorida redujo ISC y QO2, aboliendo las diferencias entre grupos. La disminución de QO2 e ISC luego de amilorida tuvo una pendiente máxima de regresión en ratas privadas de sodio y mínima en ratas cargadas de sodio. Conclusiones. El QO2 basal de ratas normales y privadas de sodio disminuye por bloqueo de ENaC. La aldosterona incrementó proporcionalmente QO2 e ISC en todos los grupos, pero el acoplamiento entre metabolismo aerobio y transporte electrogénico parece más eficiente en los animales privados de sodio.
Palabras claves. Aldosterona, colon rectal de rata, consumo de oxígeno, corriente de cortocircuito, ENaC, ingesta de sodio.
Abreviaturas
QO2 : oxygen consumption rate.
ENaC: epithelial sodium channel.
ISC : short-circuit current.
RTE : transepithelial resistivity.
Two major functions of the colon are dehydration of luminal content and regulation of fecal electrolyte excretion. Net ion absorption provides the driving force for water absorption1 and is dependent on aerobic metabolism.2 Acute hypoxia reduces net ionic transfer.3 In the rat distal colon, decreases in chloride secretion are associated with reductions in oxygen consumption (QO2),4 while stimulated chloride secretion is associated with increased QO2.5
We have previously described the relationship between chloride secretion and QO2 in rat distal colon under basal and stimulated conditions5, 6 and between electrogenic sodium absorption and QO2 in the human colon.7
Under normal conditions, electrogenic transport in distal human colon reflects mostly sodium absorption, while in rats it is due to chloride secretion, with sodium absorption occurring through an electroneutral, coupled NaCl process.1 However, like other ion transporting epithelia, the colonic epithelium is a classic target tissue for the action of mineralocorticoids.8
In the rat distal colon, elevated mineralocorticoid levels switch the major sodium absorption mechanism from electroneutral NaCl absorption to electrogenic sodium absorption. This change may be caused by sodium deprivation, which increases aldosterone secretion,9 by administration of mineralocorticoids in vivo 9, 10 or by exposure of the epithelium to aldosterone in vitro.11 The effect of incubation with aldosterone is maximal in the latest portion of the distal colon,12 also called rectal colon, or simply rectum.
Sodium deprivation increases expression of both β and γ subunits of the epithelial sodium channel (ENaC) in rat distal colon.13 Interestingly, rat rectal epithelium shows amiloride-sensitive sodium transport even in rats ratas
fed on a normal sodium diet, and its magnitude is increased by incubation with aldosterone.14 It is not clear whether changes in sodium intake that have an effect on endogenous aldosterone secretion can modify the epithelial response to exogenous aldosterone, and specifically if this response is depressed during sodium overload. However, it is known that ENaC function is disturbed in inflammatory bowel diseases, accounting in part for its diarrheal manifestation, which is mostly due to impaired sodium and water absorption.15 For example, in patients with Crohn’s disease, sodium absorption in non-inflamed portions of the sigmoid colon is impaired due to a reduced expression of the ENaC γ subunit.16 Furthermore, in the colonic mucosa from patients with ulcerative colitis, aldosterone-stimulated sodium absorption in the sigmoid colon is strongly inhibited. This is associated with reduced expression of ENaC β and γ subunits, apparently caused by tumor necrosis factor alpha.17
We are not aware of reports on whether aldosterone-induced increases in amiloride-sensitive electrogenic transport modify epithelial QO2. In the present study, we assessed the effects of aldosterone incubation on short-circuit current (ISC) and QO2 in rectal epithelia from rats submitted to control, low sodium and high sodium diets.
Material and methods
Ethics
The experimental protocol was designed according to the National Institutes of Health (USA) guidelines for animal research. It was reviewed and approved by the Committee for Animal Care and Biosafety of our Medical School.
Animals
Adult Wistar-Hokkaido male rats weighing 250 to 300 g were used. They were brought from the Medical School Animal Facility and housed at 24 ºC, one animal per cage, with a 12/12 h light/dark cycle. Animals were randomly assigned to be fed for 8 days with a standard diet, a low-sodium diet or a high-sodium diet (n = 18 for each group). All rats ate food and drank fluids ad libitum.
Diets
The control group was fed with a standard pelleted diet with a sodium content of 220 μmol/g (Cargill Co). The low-sodium group was given a diet with a sodium content of 0.7 μmol/g (pelleted Sodium Deficient Diet, # 902902, ICN Biomedicals, Inc). The potassium content of both diets was 300 μmol/g. For the control group and the sodium-deprived group, the drinking fluid was distilled water. The high-sodium group received the same diet than the control group, but the drinking fluid was saline (0.9 g/dl sodium chloride; 154 μmol of sodium per milliliter). For each animal, food and fluid consumption were estimated by weighing the remaining pellets and drinking fluid each morning. These data were used to estimate daily sodium intake.
Gas, solution and drugs
A mixture of 95% O2 and 5 % CO2 (Air Liquide, Inc) was used. The Ringer solution had the following composition: 132.8 mM Na; 4.5 mM K; 1.25 mM Ca; 1.0 mM Mg; 114 mM Cl; 24 mM HCO3; 0.8 mM HPO4; 0.2 mM H2PO4; 10 mM D-glucose; 0.5 mM β-hydroxybutirate; 2.5 mM glutamine and 10 mM D-mannose.12 When gassed to saturation, the pH of the solution was 7.40. Aldosterone and amiloride were purchased from Sigma- Aldrich. Gentamicin (Schering-Plough) was added to the Ringer solution for a final concentration of 91 μg/mL to prevent bacterial overgrowth. Aldosterone was dissolved in absolute ethanol and amiloride in dimethyl sulfoxide to yield final chamber concentrations of 10 nmol/L and 0.1 mmol/L, respectively. At the added volumes, neither ethanol (10 μL) nor dimethyl sulfoxide (25 μL) had any effect on ISC or QO2.
Surgery, dissection and mounting
Under ether anesthesia, rats were killed by thoracotomy. The abdomen and the pelvic bones were cut open to remove the whole colon, from the cecum to the anus. Segments from the pelvic portion of the colon, beyond a lymph node located at the pelvic brim – also called “late distal colon” or “rectal colon”– were used. The microdissection technique has been described before.18 Isolated mucosa preparations were obtained, cut open, gently stretched, and mounted as flat sheets in Ussing chambers.
Ussing chamber
The modified Ussing chamber used in the present experiments has been previously described.6 It was airtight and had an opening of 1 cm2. Each hemichamber had a bubble trap through which drugs may be injected, and a port for inserting a polarimetric oxygen probe (CellOx 325) connected to WTW Oxi 340 oxygen meter (WTW GmbH). The probes allowed continuous measurement of oxygen concentration and temperature. Each hemichamber had a small magnetic bar in its bottom for continuous mixing of contents when placed on a magnetic stirrer (HI 300N, Hannah Instruments). The temperature was kept at 37.0 ± 0.5 ºC by an inbuilt water jacket.
Oxygen consumption and short-circuit current determination
Oxygen meters were calibrated according to the user’s manual. Their slopes were checked before each experiment. QO2 was calculated from the rate of change in oxygen concentration, chamber volume and oxygen solubility at 37 ºC. Blankruns were performed after each experiment, to make sure that the rate of decrease in oxygen concentration was below 5% of baseline epithelial QO2.
Calomel electrodes were connected to each hemichamber through 3% agar-in Ringer bridges to record transepithelial potential difference. An amplifier, with correction for bridge asymmetry and solution resistivity, allowed passing current through Ag/AgCl2 electrodes for clamping the transepithelial potential difference at 0 mV. Experiments were performed under the short-circuit condition, except for brief releases to measure open circuit potential difference. Transepithelial resistivity (RTE) was calculated according to Ohm’s law.
Plasma sodium and serum aldosterone determination
Trunk blood was withdrawn during surgery. An aliquot was treated with calcium heparine (Calciparine) and centrifuged at 1000 rpm for 30 min. Sodium concentration was measured with an 84-11 Orion Ross sodium electrode connected to a 720 A meter (Orion Research, Inc). The remaining blood was allowed to clot. Its serum was frozen for later determination of aldosterone concentration with a coated-tube radioimmunoassay (Diagnostic Products Corporation).
Experimental procedures
In a first set of experiments, the response of epithelial samples of the three groups to amiloride was assessed. The chamber was filled with Ringer, gassed to saturation, and closed. A 30-min period was allowed for stabilization of ISC, after which baseline QO2 was measured for 30 min. Amiloride was then added and, after ISC plateaued at a lower level, a second QO2 determination was performed.
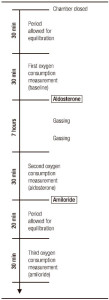
A second set of experiments evaluated the response to incubation with aldosterone. The starting procedure was the same as that just described. However, after the first QO2 measurement, aldosterone was added to both hemichambers. After 7 h, QO2 was measured by a second 30-min period. Then amiloride was added to the mucosal hemichamber and 20 min later a third QO2 measurement was performed (Figure 1).
During the aldosterone incubation period, the chamber was twice opened, gassed and closed again, to avoid excessive decreases in oxygen concentration at the end of the experiment, when two additional QO2 measurements had to be performed. Preliminary experiments performed with vehicle (ethanol) showed that gassing per se had no effect on QO2, provided that oxygen concentration in the chamber was not allowed to decrease below 5 ppm. Without aldosterone, ISC, QO2, and RTE slowly decreased, respectively, to 45%, 85% and 73% of the baseline values at the end of the 7-h period (data not shown).
Figure 1. Experimental procedure for incubation with aldosterone.
Statistical analysis
Statistical analysis was performed with Prism 5.04 for Windows (GraphPad Software, Inc). Student’s t test for paired samples was used to assess the effect of amiloride on baseline ISC, QO2, and RTE. One-way analysis of variance (ANOVA), followed by Tukey´s Multiple Comparison Test, was employed for comparing the data from the three groups. ANOVA for repeated measures was used for comparing the effect of treatments within each group. Kolmogorov-Smirnov normality test was performed to check for deviations from a Gaussian distribution. Linear regression analysis, with a check for significant deviation from linearity, was used for assessment of the relationship between ISC and QO2. Unless otherwise stated, results are expressed as mean ± SEM. Differences were deemed statistically significant at p < 0.05.
Results
Food, fluid and sodium intake
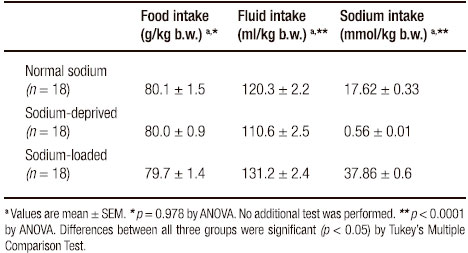
Data on food, fluid and calculated sodium intake are shown in Table 1. There was no significant difference in food intake. However, fluid intake was highest in sodium-loaded animals and lowest in sodium-deprived animals. Daily sodium intake of sodium-deprived rats (32.8 ± 0.6 mg/kg of body weight) was about 3% of sodium intake of control rats (1031.0 ± 19.2 mg/kg), while it was 214% of the latter in sodium-loaded rats (2204 ± 38.0 mg/kg).
Table 1. Daily food, fluid and sodium intake of the three groups of rats.
Plasma sodium and serum aldosterone
The results of these measurements are shown in Table 2. Serum sodium was higher in sodium-loaded animals than in both the control and the sodium-deprived group. Although the mean value of the sodium-deprived group was lower than that of the control group, the difference was not significant. Serum levels of aldosterone were markedly different, with the sodium loaded group having about a five times lower concentration, and the sodium-deprived group having a more than five-fold higher concentration than the control group.
Table 2. Plasma sodium and serum aldosterone at the time of death of the animals.
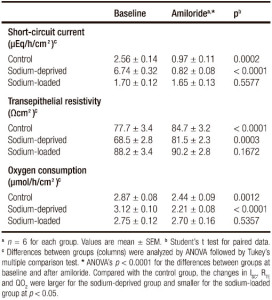
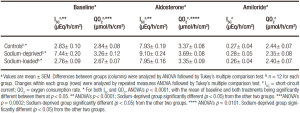
Effect of amiloride
The effects of amiloride on non-stimulated epithelial samples from the three groups are shown in Table 3. Amiloride induced significant changes in ISC, RTE, and QO2, in both the control and the sodium-deprived groups. Baseline ISC was highest in sodium-deprived rats, intermediate in control rats and lowest in sodium-loaded rats (p < 0.0001), although the difference between the two latter groups was not significant. After amiloride, the ISC of the sodium-loaded group was higher than those of the other two groups (p < 0.0001), which did not significantly differ between them.
Table 3. Effects of amiloride on short-circuit current, transepithelial resistivity and oxygen consumption of rat rectal epithelium.
Baseline RTE was significantly different (p = 0.0025), with a lower value in tissues from sodium-deprived rats and a non-significant differences between the control group and the sodium-loaded one. After amiloride, RTE was not different between groups (p = 0.1192). Also, baseline QO2 was not significantly different between groups (p = 0.0870), although there was a significant linear trend (p < 0.05) for increasing values from sodium-loaded, to control to sodium-deficient rats. After amiloride, values of QO2 were significantly different (p = 0.030) because sodium-deprived QO2 was significantly lower than sodium-loaded QO2, while control QO2 did not differ significantly from either.
While the difference in mean values of ISC, RTE, and QO2 before amiloride and after it was not always significant, the changes in all three variables induced by amiloride were highly significant (p < 0.0001), with values for each group which were significantly different from the other two groups (Table 3).
Incubation with aldosterone
Data for ISC and QO2 under baseline condition, after a 7-h incubation with aldosterone and after addition of amiloride are shown in Table 4. As observed in the first set of experiments, baseline ISC was higher in samples from sodium-deprived rats than in samples from control rats or sodium-loaded rats, but the latter two groups did not differ between them in either ISC or QO2 at the baseline measurement. At the end of the aldosterone exposure period, both ISC and QO2 were significantly raised in all three groups. However, the absolute and fractional increases in both variables were larger in the control and sodium-loaded groups. Amiloride addition abolished all differences in ISC (p = 0.6982) and QO2 (p = 0.8979).
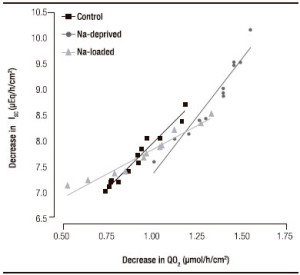
The decreases in ISC and QO2 recorded after adding amiloride to the mucosal side of the epithelia were significantly correlated in all three groups (all p < 0.0001; Figure 2). No significant deviation from linearity was found in any group. However, the slope of each line was significantly different from the other two (p < 0.0001). The line was steepest for samples from sodium-deprived rats, intermediate for samples from control rats and least steep in samples from sodium loaded rats. Thus, after amiloride addition at the end of the aldosterone incubation period, the mean absolute change in ISC per unit of absolute change in QO2 was 1.90 μEq/h/cm2 (51 μA/cm2) per μmol/h/cm2 for samples from sodium-loaded rats, 3.56 μEq/h/cm2 (95 μA/ cm2) per μmol/h/cm2 for samples from control rats and 4.58 μEq/h/cm2 (123 μA/cm2) per μmol/h/cm2 for samples from sodium-deprived rats.
Table 4. Short-circuit current and oxygen consumption rate at baseline, after incubation with aldosterone and after exposure to amiloride.
Figura 2. Regression analysis of the change in short circuit current (ISC) and oxygen consumption rate (QO2) after addition of amiloride (0.1 mM) in rectal epithelial samples incubated for 7 h with aldosterone. p < 0.0001 for all groups. Coefficients of determination (r2) were r2 = 0.963 for controls, r2 = 0.934 for sodium-deprived rats and r2 = 0.941 for sodium-loaded rats.
Discusión
We have previously estimated the fraction of epithelial oxygen consumption associated with electrogenic sodium transport in the non-stimulated human colon.7 As far as we know, this is the first study reporting the change in oxygen consumption associated with aldosterone-stimulated sodium transport of the rectal epithelium. The three groups of rats differed markedly in calculated sodium intake. Correspondingly, compared to the standard diet, the low-sodium and high sodium diets were able to significantly increase and decrease, respectively, plasma aldosterone levels.
Only the terminal portion of the colon was employed in our experiments, since more proximal portions of the rat distal colon do not show such large increases in sodium-dependent short-circuit current upon incubation with physiological aldosterone concentrations.12-14
Epithelial samples from control rats and sodium-loaded rats did not differ in baseline QO2 or ISC, although plasma aldosterone of sodium loaded rats was only about 20% of that of control rats. However, in the rectal epithelium of control rats, fed with a normal sodium diet, an amiloride-sensitive fraction of baseline ISC was measured, as previously reported by others.14 This component of ISC was absent in tissues from sodium-loaded rats. Considering the plasmatic concentration of aldosterone of each group (Table 2), these findings agree with a report stating that the threshold for an aldosterone effect in vitro – in the absence of plasmatic binding proteins – is 0.3 nM.12
On the other hand, epithelial samples from sodium deprived rats had a higher baseline ISC than the other two groups. This current was sensitive to amiloride 0.1 mM, indicating electrogenic sodium transport.9, 12 Aldosterone was present in the bath throughout the incubation period, since it has been shown that the stimulating effect of aldosterone in vitro fades if the hormone is removed from the bathing solution.12
An aldosterone concentration of 3 nM has been found quite effective to induce electrogenic transport in the rat rectal colon in vitro.12,13 However, for the experiments reported here we chose to incubate the tissues with aldosterone at 10 nM (as Inagaki et al 14 did), a concentration that still is within the range of physiological response.9, 12
One reason for this modification was that preliminary experiments showed that the response to incubation with 3 nM aldosterone was quite variable under our experimental conditions. A second and more important reason was that we wished to assess, in sodium-deprived rats, whether exposure to an aldosterone level above their plasma aldosterone concentration resulted in further stimulation of electrogenic sodium transport. This assessment could be done because plasma aldosterone in sodium-deprived rats was below 10 nM, also taking into account that free aldosterone is between 30% and 50% of total plasma concentration.19 Thus, epithelial samples from sodium-deprived rats were exposed to an aldosterone concentration up to three times higher than their free plasma level.
In fact, incubation with aldosterone significantly increased QO2 and amiloride-sensitive ISC in all three groups. The changes were least dramatic in the sodium-deprived group, whose values of both short-circuit current and QO2 were already higher at baseline than those of the control and sodium-loaded groups.
As it could have been expected from previous reports,12-14 amiloride drastically decreased ISC and QO2 after incubation with aldosterone. In this case, the decreases were larger in the sodium-deprived group. As a result, after amiloride addition at the end of the aldosterone incubation period, there was no significant difference in either ISC or QO2 between samples from the three groups.
In all groups there was a highly significant linear correlation between the reductions in ISC and QO2. We have previously shown that there are significant linear correlations between chloride secretion and ISC in the rat distal colonic epithelium.5,6 We have also found a significant linear relationship between short-circuit current and QO2 in the unstimulated human sigmoid colon.20
Previous works on the acute effect of aldosterone on electrogenic sodium transport of rat rectal epithelium in vitro studied only animals fed on a standard diet.12-14 Present results indicate that qualitatively similar effects are also observed in tissues from both sodium-loaded and sodium-deprived animals.
However, finding significantly different slopes for each experimental group of the relationship between changes in QO2 and short-circuit current was an unexpected result. Currently, we do not have an obvious explanation for this fact, which deserves further research. The different slopes indicate that, in this epithelium, the ratio between net sodium absorption and QO2 is not fixed. The metabolic cost of sodium transport was lowest in tissues from sodium-deprived animals and highest in tissues from sodium-loaded ones. This suggests that sodium deprivation induces an improvement in the efficacy of ion transport, while dietary sodium excess has the opposite effect. It is tempting to speculate that this is related to the different, chronically sustained, plasmatic aldosterone levels of each group. The relatively fast effect reported is totally or mostly non-genomic,12, 13, 21 but the differential effect for each group might depend on some complex interaction or “cross talk” between genomic and non-genomic pathways.22
In the human distal colon, amiloride-sensitive sodium absorption is impaired in inflammatory bowel diseases, in which the response to aldosterone is also blunted.15-17 In sharp contrast, amiloride-sensitive sodium absorption is preserved in a non-inflammatory condition such as diverticulosis.23 Present results suggest that the lack of electrogenic sodium absorption in epithelia from sodium-loaded rats may be entirely attributed to low endogenous serum aldosterone levels, since the response to exogenous aldosterone is preserved.
Interestingly, in the rat distal colon, aldosterone not only stimulates sodium absorption, but simultaneously decreases chloride secretion. Aldosterone activates ATP-dependent potassium channels while inhibiting Ca2+-activated potassium channels, which are, respectively, necessary for electrogenic sodium absorption and electrogenic chloride secretion.24 These contrasting effects on potassium channels have also been demonstrated in the human colon.25, 26
The concurrent stimulation of absorption and decrease in secretion makes sense as an energy-efficient mechanism to retain sodium chloride and water. In whole animals, its expected consequence is an increased dehydration of feces. Constipation is one of the symptoms of hyperaldosteronism. It is usually thought to be caused by hypokalemia, because of its inhibitory effect on intestinal transit.27 However, excessive fecal desiccation induced by the direct action of aldosterone on the colonic epithelium might also play a role.
In summary, present results show first, that ENaC blockade proportionally lowers ISC and QO2 in rectal epithelia of both rats submitted to either a normal sodium diet or a low-sodium diet, with a larger effect in the latter. No such effects were noticed in epithelial samples from sodium-loaded rats. Second, aldosterone increases ISC and QO2 of the rat rectal epithelium from animals submitted to standard, low-sodium and high-sodium diets. Third, although aldosterone-induced increases in ISC and QO2 were smaller in the sodium-deprived group, which started at higher baseline levels, this group showed the steepest relationship between change in ISC and change in QO2, suggesting that chronic sodium deprivation might increase the efficiency of epithelial sodium absorption.
Conflict of interest. None.
Sostén Financiero. Estudio subsidiado por la Secretaría de Ciencia, Técnica e Innovación Tecnológica de la Universidad Nacional de Cuyo. La participación de Karina González Otárula fue apoyada por una beca del Instituto de Ciencias Básicas de la Universidad Nacional de Cuyo.
Referencias
- Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: Mechanisms and implications for disease. Physiol Rev 2002; 82: 345-389.
- Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol 1981; 240: F357-371.
- Saraví FD, Chirino DR, Saldeña TA, Cincunegui LM, Carra GE, Ituarte LME. Chronic hypobaric hypoxia effects on rat colon in vitro sensitivity to acute hypoxia and amiloride. Dig Dis Sci 2002; 47: 1086-1090.
- Saraví FD, Cincunegui LM, Saldeña TA, Carra GE, Ibáñez JE. Indomethacin reduces short-circuit current and oxygen consumption in normal and chronically hypoxic rat colon. Acta Gastroenterol Latinoam 2006; 36: 113-124.
- Saraví FD, Cincunegui LM, Saldeña TA, Carra GE, Ibáñez JE. Increased oxygen consumption caused by cAMP- and Ca2+-mediated chloride secretion in rat distal colon. Acta Gastroenterol Latinoam 2005; 35: 13-18.
- Saraví FD, Saldeña TA, Carrera CA, Ibáñez JE, Cincunegui LM, Carra GE. Oxygen consumption and chloride secretion in rat distal colon isolated mucosa. Dig Dis Sci 2003; 48: 1767-1773.
- Carra GE, Ibáñez JE, Saraví FD. Electrogenic transport, oxygen consumption and sensitivity to acute hypoxia of human colonic epithelium. Int J Colorectal Dis 2011; 26: 1205-1210.
- Cornell JMC, Davies E. The new biology of aldosterone. J Endocrinol 2005; 186: 1-20.
- Halevy J, Budinger ME, Hayslett JP, Binder HJ. Role of aldosterone in the regulation of sodium and chloride transport in the distal colon of sodium-deprived rats. Gastroenterology 1986; 91: 1227-1233.
- Perrone RD, Jenks SL. Suppression of coupled Na-Cl absorption by aldosterone and dexamethasone in rat distal colon in vitro. Am J Physiol 1984; 246: F785-793.
- Jorkasky D, Cox M, Feldman GM. Differential effects of corticosteroids on Na+ transport in rat distal colon in vitro. Am J Physiol 1985; 248: G424-431.
- Fromm M, Schulzke JD, Hegel U. Control of electrogenic Na+ absorption in rat late distal colon by nanomolar aldosterone added in vitro. Am J Physiol 1993: 264: E68-73.
- Epple HJ, Amasheh S, Mankertz J, Goltz M, Schulzke JD, Fromm M. Early aldosterone effect in distal colon by transcriptional regulation of ENaC subunits. Am J Physiol 2000; 278: G718-24.
- Inagaki A, Yamaguchi S, Ishikawa T. Amiloride-sensitive epithelial Na+ channel currents in surface cells of rat distal colon. Am J Physiol 2004; 286: C380-390.
- Binder HJ. Mechanisms of diarrhea in inflammatory bowel diseases. Ann N Y Acad Sci 2009; 1165: 285-293.
- Zeissig S, Bergann T, Fromm A, Bojarski C, Heller F, Günther U, Zeitz M, Fromm M, Schulzke JD. Altered ENaC expression leads to impaired sodium absorption in the non-inflamed intestine in Crohn’s disease. Gastroenterology 2008; 134: 1436-1447.
- Amasheh S, Barneyer C, Koch CS, Tavalali S, Mankertz J, Epple HJ, Gehring MM, Florian P, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology 2004; 126: 1711-1720.
- Cincunegui LM, Ituarte LMI, Viera TB, Ibáñez JE, Carra GE, Saldeña TA, Saraví FD. Chronic hypoxia effects on electrogenic transport and transport-related oxygen consumption in rat distal colon. Dig Dis Sci 2008; 53: 1593-1600.
- Young WF Jr. Endocrine hypertension. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology, 12th Ed. Philadelphia, PA: Saunders, 2011:545-580.
- Carra GE, Matus D, Ibáñez JE, Saraví FD. The effects of amiloride and age on oxygen consumption coupled to electrogenic sodium transport in the human sigmoid colon. Saudi J Gastroenterol 2015; 21, en prensa.
- Booth RE, Johnson JP, Stockand JD. Aldosterone. Adv Physiol Educ 2002; 26: 8-20.
- Grossmann C, Gerkle M. New aspects of rapid aldosterone signaling. Mol Cell Endocrinol 2009; 308: 53-62.
- Osbak PS, Bindslev N, Poulsen SS, Kaltoft N, Tilotta MC, Hansen MB. Colonic epithelial ion transport is not affected in patients with diverticulosis. BMC Gastroenterology 2007: 7: 37.
- Harvey BJ, Doolan CM, Condliffe SB, Renard C, Alzamora R, Urbach V. Non-genomic convergent and divergent signaling of rapid responses to aldosterone and estradiol in mammalian colon. Steroids 2002; 67: 483-491.
- Maguire D, MacNamara B, Cuffe JE, Winter D, Doolan CM, Urbach V, O’Sullivan GC, Harvey BJ. Rapid responses to aldosterone in human distal colon. Steroids 1999; 64: 51-63.
- Bowley KA, Morton MJ, Hunter M, Sandle GI. Non-genomic regulation of intermediate conductance potassium channels by aldosterone in human colonic crypt cells. Gut 2003; 52: 854-860.
- Lee KW, Morsi A, Naga O. Endocrine disorders. In: Naga O, ed. Pediatric Board Study Guide. Cham: Springer, 2015: 403-433.
Correspondencia: Fernando D Saraví
Instituto de Fisiología, Facultad de Ciencias Médicas, Universidad
Nacional de Cuyo
Av del Libertador 50, Mendoza, MZ 5500, Argentina
Tel: (54 261) 413 5000 Extensión 2756
Cel: (54 261) 1530 56405
Fax: (54 261) 420 3288
Correo electrónico: fernando.saravi@hotmail.es / fsaravi@fcm.uncu.edu.ar
Acta Gastroenterol Latinoam 2015;45(3):203-211
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE