Osvaldo Manuel Tiscornia,1, 2, 3 Gustavo Alberto Negri,1, 2 Graciela Otero,1 Fabiana Norma López Mingorance,1, 2 Hipólito Waisman,1 Patricia Graciela Tiscornia-Wasserman1,4
1 Programa de Estudios Pancreáticos, Hospital de Clínicas, UBA,
2 Dpto de Bioquímica Clínica, Hospital de Clínicas, FFyB, INFIBIOC, UBA,
3 UCA, Pontificia Universidad Católica Argentina, Ciudad autónoma de Buenos Aires, Argentina.
4 Dpto de Patología Clínica y Biología Celular, Columbia University Medical Center, EE.UU.
Summary
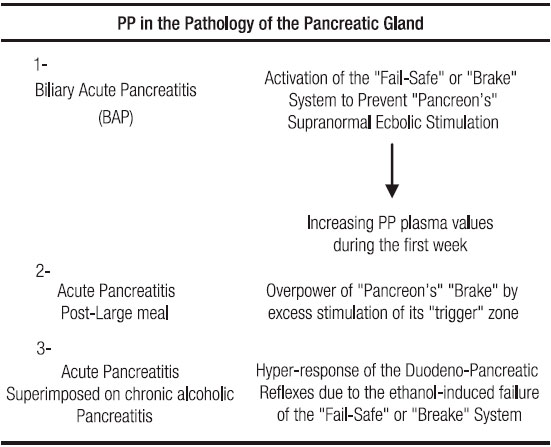
This review was prompted by the unexpected experimental finding in canines that Tissucol-induced pancreatic ductal blockade elicits Pancreatic Polypeptide (PP) release and seems to be at the basis of the beneficial effects on taurocholate-induced acute pancreatitis (AP). In the release mechanism of this regulatory peptide secreted by PP cells located in the periphery of Langerhans islets and scattered in the ductal epithelium, two neuroendocrine reflexes (NER) are involved. The «short» NER is evoked from the duodenum by an unknown component of bile-pancreatic secretion. The «long» NER is triggered by a vagovagal reflex. PP induces a depression of the intrapancreatic cholinergic tone. On the one hand suppressing, hormonally, nervous impulses discharge from the vagal nuclear complex in the brainstem. On the other, interfering paracrinically on the cholinergic transmission by acting, presynaptically, on post-ganglionic cholinergic neurons. The resulting PP-evoked fall of the intrapancreatic cholinergic tone depresses the hormone induced (secretin, CCK) pancreon’s secretory response. PP, with other agents, contributes to the «fail-safe» system or pancreon’s brake that prevents, in pancreocytes, the evolving of a «supramaximal-ecbolic-stimulation» process. The PP involvement as a modulating agent of pancreon’s reactivity is reflected by the progressive increment of its plasma values in the first week of an evolving AP episode. In the AP associated to a large meal, an overpowering of the «pancreon’s brake might have a pivotal role. In experimental and clinical chronic alcoholism, a vagal neuropathy of the Pavlov inhibitory fibers that, as a consequence, impairs the pancreon’s brake through a depression of PP secretion is at the basis of an enhanced reactivity of the duodeno-pancreatic reflexes. The latter leads to intrapancreatic cholinergic hypertonus and to Vater papilla’s dysfunction. These changes, plus an enhanced pancreocyte’s response to CCK, are at the core of acinar cell «supramaximal stimulation» with the organelle disruption that process implies. The intrapancreatic cholinergic hypertonus, the enhanced exocrine cell reactivity to CCK stimulation, and the augmented resistance to the pancreatic secretion flow at Oddi sphincter, explain the aggravating influence of chronic alcoholism on an episode of acute biliary pancreatitis. As the PP secretion, normally elicited by secretin, CCK, food and insulin hypoglycemia, is depressed in the presence of an augmented number of PP cells, as it is in the cases of chronic alcoholics, cystic fibrosis patients and, also, in dogs with pancreatic fibrosis (ductal ligation), it has been inferred, besides our postulated impairment of the Pavlov inhibitory fibers in the vagus nerves, that the defect of PP release is localized to the common final pathway of the above stimuli, probably in or near the PP cell itself.
Key words. Pancreatic polypeptide, neuro-endocrine reflexes, islet-acinar interactions.
Polipéptido pancreático: revisión de su ingerencia en los reflejos neuro-endocrinos, en la interacción acino-islote y en cambios fisiopatológicos de la glándula pancreática provocados por el etanol
Resumen
El péptido regulador PP (polipéptido pancreático) es secretado por células insulares PP, principalmente en islotes del «processus uncinatus» («gancho») del páncreas (esbozo ventral de la glándula) y, también, por células «dispersas» en el epitelio glandular. En su mecanismo secretorio se hallan involucrados dos reflejos neuroendocrinos (RNE): uno «corto» (RNE-C) y otro «largo» (RNE-L). El primero es desencadenado desde el duodeno por un componente, aún no precisado, de la secreción bilio-pancreática. El del segundo, se centra en un arco reflejo vago-vagal que da lugar a una depresión del tono colinérgico intrapancreático. Éste, por vía humoral, frena la descarga de impulsos parasimpáticos por parte del «complejo nuclear dorsal del vago». Una consecuencia bien establecida de lo precedente es la caída del rol permisivo ejercido por el tono parasimpático sobre los efectos secretorios en los «pancreones» por parte, sobre todo, de la hormona secretina. El PP, probablemente en conjunción con otros agentes, ejerce un rol de «freno» que trata de prevenir en los pancreocitos acinares el desencadenamiento de un proceso de «estimulación ecbólica supramáxima». Su rol modulador, lo demuestra, a través del incremento de su nivel en plasma en la primera semana de un episodio de pancreatitis aguda. En esta última entidad, subsecuente a una «comida copiosa», el sobrepaso de la capacidad de «freno» de la glándula tendría una influencia fisiopatogénica clave. En el alcoholismo crónico, tanto experimental como clínico, una neuropatía vagal que compromete a las «fibras inhibitorias» de Pavlov es el fundamento que explica, teniendo por pivote una depresión de la secreción del PP, la reactividad exacerbada de los reflejos duodeno-pancreáticos. Lo precedente conduce a un «hipertono colinérgico intrapancreático» y a una disfunción asociada de la papila de Vater. Estos cambios, acoplados a una respuesta incrementada de los pancreocitos a la hormona CCK, constituyen la médula del proceso que puede encuadrarse dentro del término que hemos concebido como de «estimulación supramáxima» del «pancreón». Éste suele conducir a la desorganización organelar y consecuente atrofia o muerte de la célula acinar. El hipertono colinérgico intrapancreático, la incrementada reactividad a la CCK de los pancreocitos y el aumento de la resistencia al flujo secretorio por parte de la región esfinteriana del Oddi, explican la influencia agravante del alcoholismo crónico en un episodio de pancreatitis aguda biliar. Se concluye que la secreción del péptido regulador PP, esencialmente desencadenado por la secretina, CCK, alimentos y la hipoglucemia insulínica, en la condición antes esbozada se halla deprimida; ello, paradójicamente, en presencia de un aumento en el número de su célula endocrina. Esto se constata en pacientes alcohólicos crónicos, en aquellos con fibrosis quística y también en perros con ligadura ductal. Se ha inferido que en todas estas circunstancias aparte de un compromiso de las fibras inhibitorias vagales de Pavlov («freno pancreonal») se acopla, sea próximo y/o en la propia célula efectora, un defecto en la vía final común de los estímulos antes descriptos. Una concatenación de modificaciones neuroendocrinas muy próximas a las precedentes es la que se comprueba en pacientes con insuficiencia renal crónica. Éstas logran ser mimetizadas experimentalmente en la rata efectuando la exéresis de un 85% de la masa renal. Un hallazgo a enfatizar es el que se constata en diabéticos insulino-dependientes. En esta entidad, como en el caso del alcoholismo crónico, cabe considerar una perturbada transmisión de impulsos nerviosos siguiendo el trayecto de las fibras inhibitorias vagales de Pavlov. El consecuente hipertono colinérgico intrapancreático, y quizás también hepático, este último favorecedor secretorio del factor HISS de Lautt, brindan una explicación coherente de las llamadas «curvas planas» que se aprecian en estos pacientes al efectuar una curva de tolerancia a la glucosa; también que se constaten, con cierta frecuencia, «crisis hipoglucémicas» tardías siguiendo a una ingesta de carbohidratos.
Palabras claves. Polipéptido pancreático, reflejo neuroendócrino, eje insulo-pancreonal.
This review was prompted by a series of unexpected findings with a surgical model of acute pancreatitis (AP). These were observed by the Torino group,1-3 in dogs, inducing AP by means of a Cl2Ca intraductal instillation into both segments of the pancreatic gland; the uncinate process (lower lobe) and the retrogastric (upper lobe). With this approach they had a mortality rate of 100%. But when this same protocol was performed with the additional step, 30 min later, of a Tissucol-induced pancreatic ductal blockade, the animal survival rate was of 97%. A remarkable observation was that the same results were obtained in a series of dogs4 in which the intracanalicular infusion was carried out, as before, in both pancreatic lobes but with the Tissucol-elicited ductal blockade restricted to the retrogastric segment (upper lobe). Unexpectedly, when the animals subjected to the latter approach were sacrificed 30 days later, it was striking the lack of significant histoarchitectural distortion changes and of ostensible signs of an inflammatory response in both pancreatic segments: i.e., Tissucol-blockade lobe (retrogastric) and the uncinate process.
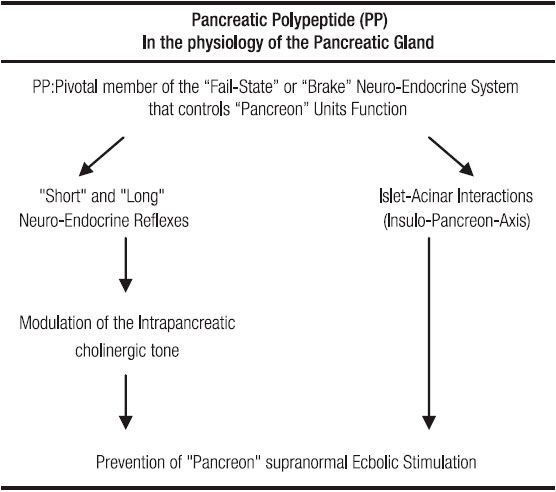
These observations induced us to postulate the hypothesis that the beneficial effects on experimental AP produced by the Tissucol filling of the ductal tree were not the consequence of a simple mechanical blockade but the result of a complex chain of reactions, consisting of the activation of neuro-endocrine reflexes (NER) and changes of the normal islet-acinar interactions. In this whole process, the regulatory peptide Pancreatic Polypeptide (PP) seemed to play a pivotal role. Indeed, in the last series of tests of the Torino group,4 a remarkable observation, detected at the level of the portal vein, was a six to ten fold increase of the PP plasma values. This, in contrast to the unmodified plasma values of bombesin, enkephalins and somatostatin. The foregoing findings prompted us to hypothesize that PP was at the center of both «long» and «short» NER, and that it played a significant role in the complex endocrine-exocrine interactions. Through both mechanisms, PP might be able to attenuate the AP lesions, the beneficial effects probable linked to a reduced pancreon5 secretory activity. The latter probably as the consequence of a PP-evoked depression of the intrapancreatic cholinergic tone blocking of CCK effects and inhibition of acinar cells enzymes exocytosis. (Figure 1, 2 and Table 1)
The adrenergic component of the autonomic nervous system also influences PP secretion. Thus, beta-blockade inhibits PP release. In contrast, alpha-blockade enhances PP secretion. The latter might be related to the raised PP plasma values observed after celiac ganglionectomy. The latter finding seems to provide a new support, as therapeutic measure, to the local anesthesia of this autonomic structure in cases of an AP episode.
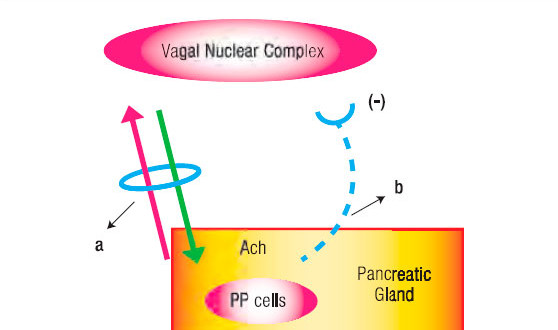
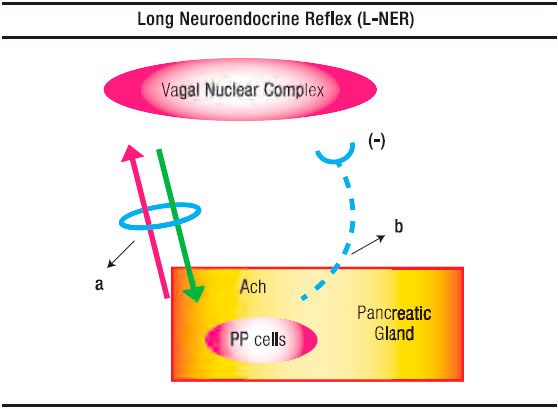
Figure 1. Schematic Representation of the Long Neuro-Endocrine Reflex. a. Vagus Nerve with its afferent and efferent fibres. b. Inhibitory hemiloop of the reflex arc that blocks the vagal nucleus discharges.
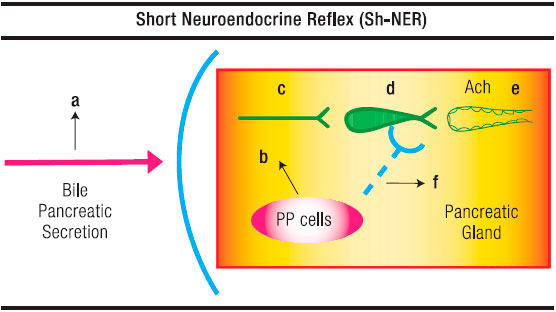
Figure 2. Schematic Representation of the Short-Neuro-Endocrine Reflex. a. Triggering in the duodenum by an unknown bile-pancreatic secretion component of PP from the PP cells; b. Preganglionic fibre; c. lntrapancreatic cholinergic neuron; d. Pancreon unit; e. Blocking of Ach discharge by pancreatic polypeptide at presynaptic level.
Table 1. Main features of PP involvement in the physiology of exocrine pancreas.
PP is also involved in the interactions that evolve within the insulo-pancreon-axis interactions. Firstly, PP might exert a local trophic effect on exocrine pancreas, protecting the PP-rich lobe of the gland (uncinate process) from atrophy in diabetic patients. Secondly, following a meal, as the increasing insulin stimulates digestive enzyme synthesis in the peri-insular cells, PP, together with somastotatin, might inhibit inmediate exocrine release of stored enzymes.
Preliminary observations, unpublished Torino findings4 in rats, of a significant drop in mortality rate of taurocholate-induced AP following the infusion of PP seems to warrant the performing of further tests in order to elucidate whether or not this regulatory peptide is, in fact, a valuable preventive and/or therapeutic agent in acute inflammatory episodes of the pancreatic gland.
Main features of pp release and action
PP is a 36-aminoacid polypeptide, structurally related to peptides that include peptide YY (PYY) and neuropeptide Y (NPY).9
Pancreatic polypeptide is a hormone released from the «F» or «PP» cells. These are normal constituents of the Langerhans islets, precisely of those derived from the ventral pancreatic bud. Because of this singular embryonic derivation, expression of PP inmunoreactivity has been proposed as a marker of the ventral pancreatic bud origin. The distribution of the PP cells in the pancreatic gland is inhomogeneous. They are located predominantly in the periphery of the pancreatic islets. A minority are found scattered among the cells of both the acinar and centroacinar-ductal segments of the pancreon unit.5
As we have already pointed out, PP cells develop solely in the ventral pancreatic bud during early embryogenesis. At week 5-7 post-gestation, complex morphologic events take place. The ventral bud rotates around the embryonic foregut to occupy its final position as the posterior pancreatic head and uncinate process. In the adult pancreas, over 90% of the PP cells are located on the posterior portion of the pancreatic head, an area that corresponds to only 15 % of the entire pancreatic volume.
PP secretion is stimulated by «chew and spit» sham feeding. This response is abolished by vagotomy and atropine. Hypoglycemia, whether induced by insulin or tolbutamide, strongly stimulates PP secretion. But the most powerful stimulus for the release of this regulatory peptide is a acetylcholine (Ach). It should also be pointed out that peptides, such as CCK, bombesin and neurotensin may activate receptors on nerve cells and/or fibers and stimulate the PP cells.10-15
A physiologic detail related to PP release from the digestive tract is that distention is an efficacious stimulus in man’s stomach and duodenum.10 Indeed, distending the gastric fundus raises PP plasma values. This reflex is abolished by the selective denervation of this stomach region (Figure 1).
A chemical stimulus is also operative to release PP. This has been shown by the presence of food in the gut10 and by the infusion of bile and pancreatic proteases into the duodenal lumen. The latter constitutes the basis of an «entero-pancreatic-inhibitory reflex» in which, according to Owyang et al16 PP plays a pivotal role. These authors have also stated that bursts of pancreatic and biliary secretion into the duodenum during late phase 2 and early phase 3 of the intestinal migrating complex and the associated increase in plasma PP are causally related. This contention gets support in their finding that perfusion of the duodenum with bile-pancreatic juice stimulates the release of PP into the circulation. This regulatory peptide might be responsible for the marked suppression of pancreatic and bile acid output during phase 4 of the intestinal migrating motor complex (Figure 2).
Owyang et al16 have concluded that an undefined factor in bile-pancreatic secretion, unrelated to pH, osmolarity or protein, is responsible of the PP release. According to their view, a cholinergic reflex is involved. Our group17 has also postulated, in rats, that the inhibition of bile-pancreatic secretion induced by its infusion into the duodenum was the result, at least partially, of a duodeno-pancreatic neural inhibitory reflex.
As regard the intimate mechanism of the above reflex, Jung et al18 have provided evidence that PP, in rat pancreatic slices, inhibits potassium-stimulated amylase release but has no effect on the Ach or the octapeptide-elicited secretion of the enzyme. This suggests that PP exerts its action through neural elements, that it acts, essentially, on post-ganglionic cholinergic neurons. The site of inhibition of the cholinergic transmission might be pre-synaptic, between neuron and acinar cells. These effects of PP are, in fact, the first demonstration of a hormone suppressing pancreon’s enzyme secretion through an interfering of the intrapancreatic cholinergic transmission.
The above-described PP-evoked changes are duplicated by pancreastatin, methionine-enkephalin and somatostatin.19 These agents provide a fail-safe system that prevents the pancreocytes «supramaximal ecbolic stimulation». Adler et al9 have stressed that somatostatin-28, the same as PP, belongs to what we have described as a pancreon’s brake8 or as a restraining rein on the physiologic interplay that modulate the exocrine pancreatic secretory process.
As shown later, somatostatin-14, released from the islet’s mantle D-cells, the same as PP, secreted from the «F» or «PP» cells in the Langerhans islets periphery, contribute to modulate the pancreon’s secretory process through influences exerted primarily upon the peri-insular pancreocytes (Figure 3).
During an episode of AP, the pancreatic gland tries to prevent overstimulation of the acinar cells. The testimony of this, in patients, is a progressive increment in the PP plasma values during the first week of an evolving acute inflammatory episode.20 (Table 2).
In the results reported by Torino et al1-3 in which the Tissucol filling of the pancreatic ducts, subsequent to the Cl2Ca-evoked of an AP, allowed the survival of dogs that otherwise might be dead within a 36hs period, the elevated PP plasma values probably disclose that a NER has been triggered. The Tissucol-evoked rising of the PP plasma levels probably occurred superimposed upon spontaneous-enhanced secretion of this regulatory peptide.4
Table 2. Involvement of PP in different types of acute pancreatitis.
The pathways of the long neuroendocrine reflex
Having outlined the main features of the short NER, in fact equivalent to our previously described negative duodeno-pancreatic-reflex,8, 21, 22 and the one postulated by Owyang et al16 as an entero-pancreatic-inhibitory reflex in the feedback control of the exocrine pancreatic secretion, it remains to be analyzed the long NER (Figure 1 and 2). The latter consists of two distinctly hemiloops. The first one runs from the vagal-nuclear-complex in the brain-stem up to the PP cells which are located in the periphery of the Langerhans islets and scattered in the exocrine pancreas. The hemiloop that completes the feedback circle pivots around the circulation of PP from the pancreatic gland up to the vagal-nuclear-complex. The nerve impulses that flow the former hemiloop run, initially, in the gastric branches of both vagal trunks. Subsequently, through what we have conceived as a neural-plexual-freeway, that has as a background the gastroduodenal myenteric and submucosal neural networks.23 After traversing the pyloru or bypassing it following the pathway of the hepatic branch of the left vagus, reaching afterward the neural plexus of the enteric “freeway” at the first duodenal segment, the neural vagal impulses follow the nerve fibers that jump, in rich density, the duodeno-pancreatic cleft. This duodeno-pancreatic linking takes place primarily in the region comprised between the pylorus and the peri-Vaterian región.24-34 Confirmation that this is precisely the pathway followed by the first hemiloop of the long NER is provided by the experimental observation, in dogs, that duodeno-pancreatic disconnection or duodenectomy29 diminishes, on the one hand, the hypoglycemia-induced PP release and, on the other, the hormonal PP response to a meal.
As for the second hemiloop of the long NER, the one that pivots around the blood stream PP flow from the pancreatic gland up to the vagal-nuclear-complex in the brain-stem, the group of Taylor29 has given evidence that this regulatory peptide does indeed enter in contact with the vagal-nuclear-complex. The latter is achieved at the level of the area postrema and the tractus solitarius nucleus, two regions of the central nervous system that lack a blood barrier. Once in the vagal-nuclear-complex binds to specific receptors and inhibits the neuron discharge of the dorsal motor nucleus. The consequence is a depression of exocrine pancreatic secretion due to a fall of the intrapancreatic cholinergic tone. The latter, as we have already pointed out, normally exerts an intricate interaction with the hormonal system upon the pancreon’s secretory process.5 (Figure 1).
From all the preceding facts, it is evident that the long as well as the short NER are important components of a fail-safe system or, in other words, of a brake mechanism that, under physiologic circumstances prevents the noxious consequences upon the acinar pancreocytes which are attached to abrupt episodes of supramaximal-ecbolic-stimulation.33, 34 (Figure 1, 2 and Table 1).
When the above-depicted fail-safe or brake system is overpowered, as for example following a large meal rich in proteins, fats and alcohol, an episode of AP can be evoked.
In the sixties, we have pinpointed this clinical entity as an AP due to an over activation of the «trigger» zone (PV-D) of the exocrine pancreas (“pancreon” units).5, 8, 33, 34 (Table 2).
Analysis of the interactions between ethanol intoxication and neuro-endocrine reflexes
A physiopathogenic feature to emphasize is that of the probable involvement of long NER in the mechanism of exocrine pancreatic secretion depression induced by an acute i.v. ethanol injection. This phenomenon was unexpectedly observed in a long series of tests performed in humans, dogs and rats.35-37 As this ethanol-elicited inhibition on secretin or secretin + CCK-evoked exocrine pancreatic secretion was prevented by previous vagotomy or atropinization, we hypothesized that ethanol was essentially an activator of the classical known «Pavlov fibers» (vagal inhibitory).35-37
A remarkable subsequent observation was that chronic ethanol intoxication induced a reversal of the exocrine pancreatic secretion inhibitory response evoked, during the nonalcoholic stage, by an acute ethanol administration.36 This finding was interpreted by us as the consequence of alcohol-elicited impairment of the normal physiologic «brake» system in the control of exocrine pancreatic secretion by the pancreon units.
The presumption was that an ethanol-induced vagal neuropathy was responsible of this change. The latter preferentially impinging upon the Pavlov fibers (inhibitory), precisely the ones resulting in the release of PP and perhaps other members of the pancreon’s fail-safe system, like beta-endorphins, substance P and somatostatin.
The release of PP and/or beta-endorphin evoked during the nonalcoholic stage by an episode of acute ethanol intoxication might explain the remarkable divergent changes of gastric and exocrine pancreatic secretion. Indeed, under the above circumstances, there is an enhancement of gastric acid secretion and a simultaneous inhibition of exocrine pancreatic secretion.38 Suggestively, similar secretory responses, as the above outlined, are observed with the administration of either beta-endorphin38 or PP.41
As to the postulation of an alcohol-evoked impairment of the Pavlov fibers, the vagal neuropathy described in humans by Duncan et al42 and the degeneration of intrapancreatic nerve fibers shown by Berger and Feher in mice,39 provide a suggestive support to this contention. Moreover, the secretory studies, in dogs, by Schmidt et al40 add further confirmation to our previously stated assumption of an ethanol-elicited gradual disappearance of an exocrine pancreatic secretion inhibitory factor.
The Hajnal et al43 report, in humans chronic alcoholics, of a lack of PP response following an acute ethanol or wine administration superimposed to a test meal, undoubtedly offers an additional support to our hypothesis above outlined.
The loss of involvement of the Pavlov vagal inhibitory fibers induces the phenomenon of neural decentralization and the consequent enhanced reactivity of the peripheral antro-duodeno-pancreatic reflexes, and as a result of that, an increased intrapancreatic cholinergic tone.33, 34 In fact, the idea of an evolving supranormal-ecbolic-stimulation of the pancreon units secondary to the loss of the negative component of pancreas innervation (PP. endorphins, etc) is at the core of our postulation concerning the physiopathogenesis of alcoholic pancreatitis.34 (Table 2).
That chronic alcoholism is indeed associated with an increased reactivity of the entero-pancreatic reflexes, is supported by Brugge et al44 findings. These authors have observed, in man, that: a) basal duodenal trypsin output and the interdigestive duodenal contraction rate are higher in chronic alcoholics that in controls; b ) chronic alcoholics have an increased post-prandial trypsin secretion compared to nonalcoholics; c) alcohol-fed and nonalcoholics show similar post-prandial increments in plasma levels of gastrin and CCK; d) when compared to nonalcoholics, chronic alcoholics display a remarkable lack of PP increments in respect to a test meal or test solutions; e.g., glucose, ethanol, wine.
Yamasaki et al45 have reported, in monkeys, that chronic ethanol administration induces papillary dysfunction and exocrine pancreas hyper secretion. Both might play a role in the observed raised pancreatic ductal pressure and the microscopic signs of acinar pancreocytes hyper function.
It is our postulation that in the above-described changes the ethanol-evoked impairment of the pancreon’s brake, primarily of PP must play a crucial role.
A feature to emphasize is that a similar sequence of events develops in uremia. Indeed. Owyang et al46 have shown, in patients with chronic renal insufficiency, that both intraduodenal mannitol and i.v. CCK evoke an ecbolic hyperresponse of the exocrine pancreas. A closely similar finding was reported in rats in which the renal mass had been 85% surgically reduced.47 This unexpected exocrine pancreatic secretion change response was attributed to an autonomic nervous imbalance characterized by a predominant cholinergic prevalence.
In the above clinical and experimental setting, it seems logical to presume an impairment of the pancreon’s brake with a failure centered in the synthesis and/or release of PP.
Another observation to be stressed is that the same fall of PP secretion that we have commented upon in chronic alcoholic patients have been shown in patients with cystic fibrosis and in dogs with pancreatic fibrosis following ductal ligation.48, 49
A feature to emphasize in all these entities is that the depression of PP release occurs in the presence of an augmented number of PP cells in both the islets and the exocrine parenchyma.
The above observation prompts the speculation if in the chronically inflamed gland the PP cells produce less hormone than normally or, contrariwise, release less in response to different stimuli.
As the absolute secretion normally elicited by secretin, CCK, food and insulin hypoglycemia is similarly affected, one is tempted to assume that the defect of PP release in chronic pancreatitis is localized to the common final pathway of these stimuli, probably in or near the PP cell itself.
The above discussed PP secretion failure is probably at the basis of the enhanced intrapancreatic cholinergic tone characteristic of chronic alcoholics. In these patients it might explain the supranormal ecbolic pancreatic response following the intraduodenal infusion of a CCK releaser (oleic acid) and; besides, of its normalization by previous atropinization.50
The above findings justify our postulation that chronic alcoholism sensitizes the trigger zone (PV-D) of the pancreatic gland.22, 31, 33, 51 This notion helps to explain why these patients are more prone to develop AP episodes especially following the ingestion of large meals, rich in protein and fat (CCK releasers), phenomenon that is potentiated when associated to alcohol ingestion (Table 2).
Adrenergic innervation and pp secretion
Besides the already discussed interactions that evolve between PP and the pancreatic cholinergic innervation, it is worth considering those that relates to the adrenergic component of the autonomic nervous system. In that sense, several studies have shown that beta-blockade inhibits PP release, while, contrariwise, alpha-blockade enhances PP secretion.
The raised PP plasma values observed after celiac ganglionectomy might be related to the interruption of alpha adrenergic influences; according to Larson et al52 celiacectomy may be considered a sort of alpha-adrenergic-blockade.
In relation with the fore mentioned innervation features, it should be pointed out that celiac ganglionectomy does indeed affect the nerve terminals and the catecholamine content of the pancreas: norepinephrine drops by 90%, while epinephrine and dopamine fall by 50 to 70%.7, 9, 52, 53 Blocking of the celiac ganglia by local anesthetics might trigger a rising of the PP plasma values. If this is confirmed, it could mean a new support to justify this procedure in cases of AP. In this clinical setting, celiac ganglion anesthesia would constitute a therapeutical measure not only to relieve pain but also an efficacious method to reduce the intrapancreatic cholinergic tone.
PP Involvement in the pancreatic trophic changes
When the NER are disrupted or when a diabetic syndrome has fully developed, islet-acinar (insulo-pancreon-axis) alterations are induced that, themselves, evoke trophic changes in the pancreatic gland.
The former situation is represented by our results in rats subjected to either a transection and reanastomosis of the peri-Vaterian region or to a resection of supra- Vaterian duodenal segment.24 The pancreatic head wet weight increase observed under the above circumstances is probably related to an enhanced PP concentration in the pancreatic gland. This probably as a result of the interruption of the cholinergic impulses flowing through the hypothetical of the network of the enteric autonomic nervous system, characterized by us as a sort of neural-plexual-freeway.23 The main support of this contention is the Raher et al’s report. These authors have speculated that PP cell, located predominantly (90%) in the dorsal of the pancreas may exert a local trophic effect. This property might protect the PP-rich lobe of the gland from atrophy in diabetic patients. These statements of Raher et al13 are based on their observations at an autopsy level. Indeed, in insulin-dependent diabetics (IDO) it was remarkable that the pancreas was markedly decreased but at the expense of an almost selective atrophy of the lobe poor in PP cells. A similar finding, although of a lesser magnitude, was appreciated in non-insulin dependent diabetics (NIDD) patients. These findings are in keeping with those of Greenberg et al54 observations in rats in the sense that PP increase DNA synthesis in pancreatic acinar cells. They are also coherent with the microscopic detail that PP cells present long cytoplasmic processes in contact with the exocrine cells. The above features fit coherently with the suggestive Oria’s55 observations that the pancreatic hook segment, the one, located around the mesenteric vessels, precisely the richest one in PP cells, is frequently spared in the severe episodes of necrotizing AP.
Concerning diabetics, Will et al56 have reported that these patients with signs of autonomic neuropathy reveal an impaired PP plasma response following insulin- induced hypoglycemia. That is to say a clinic-pathological situation closely resembling that of chronic alcoholism.
Other features of the pp involvement in the insulo-pancreonaxis interaction
The Raher et al13 findings bring into focus the interesting phenomenon of the islet-acinar-axis interactions.57 Some of them are unequivocally exerted by the PP cells located in the periphery of the Langerhans islets.
Nakagawa et al58 have recently concluded that the insulo-pancreon-axis works cytotrophically (e.g., increasing cell growth and enzyme synthesis) via insulin over the long-term, whereas somastotatin and/or PP provide a means to rapidly regulate the suppression of enzyme release from the acinar cells.
The above speculation is supported by the well known and accepted histologic phenomenon centered on the presence of peri-insular halo that has been accepted as an expression of more synthesis and less secretion of enzymes in the peri-insular region.
Immediately after a meal, the increased insulin could stimulate digestive enzyme synthesis in the peri-insular cells, whereas local somastotatin and PP may inhibit immediate exocrine release of stored enzymes.
Chronic pancreatitis and pp
In this review it seems appropriate to make reference to the fact that PP administration ameliorates the hepatic insulin resistance that has been demonstrated in chronic pancreatitis. Seymour et al59 have reported that PP secretion is impaired in patients with severe chronic pancreatitis and that a strong correlation exists between test meals demonstrated PP deficiency and hepatic insulin resistance. They have also shown that chronic PP treatment increases insulin receptor concentration in hepatocytes membranes from chronic pancreatitis rats and also improves glucose tolerance after enteral administration of dextrose.
According to Brunicardi,59 in the diabetic syndrome associated with chronic pancreatitis or the Whipple procedure (pancreatogenic or type 3 diabetes), a deficiency of PP may play an important role. This speculation should be coupled to that of a glucagon deficit originally suggested by Bank.57
To the impairment of both PP and glucagon it should be added that of a beta-cell hypo function. The latter would be consequence of a previous period of cholinergic-elicited beta-cell hyper stimulation that has been put in evidence by Patto et al60 in chronic alcoholic patients.
Berger and Feher39 have shown, in mice, after alcohol feeding, the degeneration of the intrapancreatic nerves which are an important source and locus of action of PP. These immunohistochemistry findings provide support to our contention that alcohol feeding does indeed impair the normal physiologic brake that modulates exocrine pancreatic function. This change, as we have already pointed out, might be at the basis for the increased reactivity of the trigger (PV-D) and of the pancreon units that characterize the earlier stages of alcohol-evoked chronic pancreatitis.
Final comments
All the forementioned data provide substantial basis to justify our earlier contention that the Tissucol-evoked beneficial effects upon the dog’s Cl2Ca-elicited AP lesions are related to the enhancement of normal islet-acinar cells interactions and triggering of the long NER from the intrapancreatic ductal tree.
The latter assumption seems to get support in the results observed with the exogenous PP administration in rats subjected to taurocholate-induced AP.4 Indeed, when this regulatory peptide was subcutaneously injected every 8 h preceding the AP episode, the animal mortality rate dropped from 100 to 50%. When PP was injected for 7 days following the taurocholate-induced pancreatic inflammatory lesion, animal mortality rate was of 52%, but when the above-described approaches were associated, the mortality rate fell significantly to a level of 15%.
From all the above gathered information and discussion, it seems logically warranted to perform tests with PP in order to elucidate whether or not this regulatory peptide is, in fact, a valuable preventive and/or therapeutic agent when an acute inflammatory episode is suspected.
Referencias
- Torino F, Romero O, Garaycoechea M, Garcia H, Schenk C, Hojman D, Tiscornia OM, Perera J. Pancreatitis aguda necro-hemorrágica experimental. Rev Argent Cirug 1985;99:276-279.
- Torino F, Fagniez P, Rotman N, Gil O, Garaycoechea M, García H, Schenk C, Hojman D. Pancreatite aigue necrotico-henorragique experimentale chez le chien. J Chir (Paris) 1989;2:88-90.
- Torino F, Gil O, Garaycoechea M, García H, Casavilla A, Chenk C, Hojman D. Pancreatic duct occlusion in the management of acute necrotizing pancreatitis in a canine model. Mt Sinai J Med 1989;56(2):79-82.
- Torino F, Garaycoechea M, Garcia H, Casali FJ, De Luca E, Viviani M, Sarsotti ME, Tiscornia OM. The beneficial effects of canine pancreatic duct occlusion with Tissucol in acute experimental pancreatitis is linked to a neuro-endocrine reflex that releases pancreatic polypeptide (PP). To be Submitted.
- Dreiling D, Tiscornia O M. Tests of Pancreatic Function. In: Scientific Foundations of Gastroenterology. London: Publisher: W Heinemann Medical Books, 1980:591-601.
- Tiscornia OM. Controle nerveux cholinergique du pancréas. Biol Gastroenterol (Paris) 1976;9:225-270.
- Tiscornia OM. The neural control of exocrine and endocrine pancreas. Am J Gastroenterol 1977;47:541-560.
- Tiscomia OM, Dreiling D, Yacomotti J, Kurtzbart R, De la Torre A, Farache S. Neural control of the exocrine pancreas. An analysis of the cholinergic, adrenergic and peptidergic pathways and their positive and negative components I, Neural Mechanisms. Mt Sinai J Med 1987;54:366-383.
- Adler G, Nelson DK, Katschinski M, Beglinger Ch. Neurohormonal control of human pancreatic secretion. Pancreas 1995;10(1):1-13.
- Schwartz T W. Pancreatic polypeptide: a hormone under vagal control. Gastroenterology 1983;85:1411-1425.¬
- Putnam W S, Liddle R A, Williams J A. Inhibitory regulation of rat exocrine pancreas by peptide YY and pancreatic polypeptide. Am J Physiol 1983;256:G698-G703.
- Sundler F, Bötcher G. Islet innervation with special reference to neuropeptides.Ed E Samols. In: The Endocrine Pancreas. New York, Raven Press Ltd,1991:29-52.
- Raher J, Wallon S, Lefevre A, Gepts W, Haot J. The pancreatic polypeptide cells in the human pancreas. The effects of age and diabetes. J Chem Endocr and Metab1983;56(3):441-444.
- Prinz R A, El Sabbagh H, Adrian T E, Bloom S, Gardner L, Polak J, Inoukuchi J, Bishop A F, Welbourn R. Neural regulation of pancreatic polypeptide release. Surgery 1983;94(6):1011-1018.
- Zaccaria M, Giordano G, Pasquali C, Ragazzi E, Zevjani M, Valentini P, Scandellari C. Effects of pirenzepine on plasma insulin, glucagon and pancreatic polypeptide levels in normal man. Eur J Clin Pharmacol 1985;27:701-705.
- Owyang Ch, Achen-Karam SP, Vinik A. Pancreatic polypeptide and intestinal migratory complex in humans. Gastroenterology1983;84:10-17.
- Tiscornia OM, Dreiling D. Is basal bile-pancreatic juice influenced by gastric juice diversion in the rat? Mt Sinai J Med 1986;53:368-376.
- Jung G, Louie D S, Owyang Ch. Pancreatic polypeptide inhibits pancreatic enzyme secretion via a cholinergic pathway. Am J Physiol1987;253:G706-G719.
- Karkl-Heinz H, Louie D S, Tatemoto K, Owyang Ch. Pancreastatin inhibits pancreatic enzyme secretion by presynaptic modulation of acetylcholine release. Am J Physiol1992;262:G113-G117.
- De Diego-Carmona J A, Molina-Trigueros L, Hamelin M, Sanchez A, Lopez J, Correas L, Represa J, Velazquez L. Comportamiento de la secreción de polipéptido pancreático por la célula PP durante la pancreatitis aguda. Rev Esp Enf Ap Digest 1985;67(2):160-164.
- Tiscornia O M, Martinez J L, Sarles H. Some aspects of humans and canine pancreas innervation. Am J Gastroenterol 1976;66:353-361.
- Tiscomia O M, Sarles H, Voirol M. Evidences for duodeno-pancreatic reflexes and an anti-CCK factor with lidocaine infused intravenously and sprayed topically on pancreatic papilla in nonalcoholic and alcohol-fed dogs. Am J Gastroenterol 1976;66:221-240.
- Tiscornia O M, Hamamura S, Lehmann E S de, Gonzalez E, Vaccaro M I, Otero G, Cerini C, Waisman H. Una visión de la inervación autonómica gastro-entero-bilio-pancreática. El concepto de «Pista-Plexual-Entérica». Pren Med Argent 1998;15:494-503.
- Tiscomia O M, Celener D, Cresta MA, Perec C, Tumilasci O, Dreiling D. Trophic and anti-trophic circuits controlling pancreatic weight in the rat Mt Sinai J Med 1996;53:343-355.
- Anglade P, Michel C, Roze CI. Intrinsic nerves of the pancreas after celiac and superior mesenteric ganglionectomy in rats. A morphologic study of acetylcholinesterase activity and catecholamine histofluorescence. Pancreas 1987;2:568-577.
- Kirchgessner A L, Mawe G M, Gershon M D. Evaluation of the activity of chemically identified enteric neurons through the histochemical demonstration of cytochrome oxidase. J Comp Neurology 1990;301:1-14.
- Kirchgessner A L, Gershon M D. Innervation of the pancreas by neurons in the gut. J of Neuroscience 1990;10:1626-1642.
- Kirchgessner A L, Gershon M D. Presynaptic inhibition by serotonin of nerve-mediated secretion or pancreatic amylase. Am J Physiol1995;268:G339-G345.
- Okumura T, Pappas Th, Taylor I L. Pancreatic polypeptide microinjection into the dorsal motor nucleus inhibits pancreatic secretion in rats. Gastroenterology 1995;108:1517-1525.
- Miyasaka K, Masuda M, Funakoshi A. Involvement of S-hydorxytriptamina (5-HT) neurons in regulation of basal pancreatic secretion in conscious rats. Pancreas. 1996;13(4):451(A).
- Tiscornia O M, Hamamura S, Cresta M A, Lehmann E S de, Negri G, Gonzalez E, Tiscomia-Wasserman P. Duodenal peri-Vaterian autonomic nervous center in the rat: indirect evidence given by papillar anesthesia, Vaterian exclusion, supra and infra-Vaterian transection and reanastomosis, celiac ganglionectomy and distal bilateral vagotomy. Am J Gastroenterol 1993;88(9),155(A).
- Poulsen J, Delikaris P, Lovegreen N A, Schwartz T W. Impaired pancreatic innervation after pyloric transection in dogs. Scand J Gastroenterol1983;18:17-22.
- Tiscornia O M, Perec C, Celener D, Cresta M A, Tumilasci O, Lehmann E S de, Dreiling D. The relationship of hyperactivity of the duodenal autonomic nervous brain and enhanced pancreon secretory response to CCK in chronic alcoholics. Mt Sinai J Med 1984;51:650-663.
- Tiscornia O M, Dreiling D. Physiopathogenic basis of alcohol pancreatitis: supranorinal ecbolic stimulation of the «pancreon» units secondary to the loss of the negative component of the pancreas innervation. Pancreas 1987;2:604-612.
- Tiscornia O M, Gullo L, Sarles H. The inhibition of canine exocrine pancreatic secretion by intravenous ethanol. Digestion 1973;9:231-240.
- Tiscornia O M, Palasciano G, Sarles H. Effects of chronic ethanol administration on canine exocrine pancratic secretion (Further Studies). Digestion 1974;11:172-182.
- Tiscornia O M, Iovana J, Tumilasci O, Perec C, Cresta M A, Celener D, Dreiling D. Effects of intravenous ethanol on basal bile-pancreatic secretion in nonalcoholic and alcohol-fed rats. Mt Sinai J Med 1990;57:353-361.
- Tiscornia O M, Palasciano G, Dzicniszcwski J, Sarles H. Simultaneous changes in pancreatic and gastric secretion by acute intravenous ethanol infusion. Am J Gastroenterol 1975;63:383-395.
- Berger Z, Feher E. Degeneration of intrapancreatic nerve fibres after chronic alcohol administration in mice. Int J Pancreotol. 1997;21(2):165-171.
- Schmidt N, Devaux M A, Bradzinski T M, Sarles H. Disappearance of an inhibitory factor of exocrine pancreas secretion in chronic alcoholic dogs. Scand J Gastroenterol. 1982;17:761-768.
- Mc Tigue D, Rogeos R. Pancreatic polypeptide stimulates gastric acid secretion through a vagal mechanism in rats. Am J PhysioJ. 1995;269:R983-R987.
- Duncan G, Lambee D G, Johnson R H, Whiteside E A. Evidence of vagal neuropathy in chronic alcoholics. Lancet 1980;2:1053- 1057.
- Hajnal P, Flores C, Valenzuela J E. Pancreatic secretion in chronic alcoholics. Effects of acute alcohol or wine on response to a meal. Dig Dis& Sci 1993;78(1):12-17.
- Brugge W R, Burice C A, Brand D L, Chey W. Increased interdigestive pancreatic trypsin secretion in alcoholic pancreatic disease. Dig Dis & Sci. 1985;30:431-439.
- Yarnasaki K, Ckasaki K, Sakamoto Y, Yamamoto Y, Okada T. Effects of ethanol on the motility of papillary sphincter and exocrine pancreas in the monkey. Am J Gastroenterol1993; 88(12):2078-2083.
- Owyang C, Miller L J, Di Magno E P, Mitchell J C, Go V L. Pancreatic exocrine function in severe human chronic renal failure. Gut. 1982; 23:357-361,1982.
- Fleischer K, Kasper H. Exocrine pancreatic function in uremic rats. Acta Hepat Gastroenterol1974;21:398-403.
- Stern H, Davidson G P, Kirubakaran C P, Reutasch J, Hansky J. Pancreatic polypeptide secretion as a marker for disturbed pancreatic function in cystic fibrosis. Dig Dis & Sci 1983;28(10):870-873.
- Inoue K, Watson L C, Thompson J. Reduction of post-prandial release of pancreatic polypeptide after development of pancreatic fibrosis. Surg Gynecol & Obst 1982;154:699-703.
- Voirol M, Bretholz A, Laveque O, Laugier R, Tiscornia O M, Sarles H. Atropine-induced inhibition of the enhanced CCK release observed in alcoholic dogs. Digestion 1974;14:174-178.
- Tiscomia O M, Celener D, Vaccaro M I, Waisman H. Pancreatitis aguda: Hipótesis fisiopatogénica de la necrosis grasa. Medicina1988;48:530-542.
- Larson G M, Sullivan H W, O’Dorisio Th. Surgical sympathectomy increases pancreatic polypeptide response to food. Surgery1985; 98(2):236-242.
- Brunicardi F Ch, Druck P, Seymour N T, Su Sun Y, Gingerich R, Darlush E, Andersen D K. Splanchnic neural regulation of pancreatic polypeptide release in the isolated perfused human pancreas. Amer J of Surgery 1989;157:52-56.
- Greenberg G R, Mitznegg P, Bloom S R. Effect of pancreatic polypetide on DNA synthesis in the pancreas. Experientia 1997;33:1332-1334.
- Oria A. Personal communication. 1998.
- Witt N S, Vinik A L, Sive A, Van Tonder S, Lund A. Impaired pancreatic polypeptide responses to insulin-induced hypoglycemia In diabetic neuropathy. J Clin Endocrinol & Metabol 1980;50(3):445-449.
- Bank S. Pancreatic endocrine-exocrine relationships in health and disease. Scand J Gastroenterol 1972;7:503-507.
- Nakagawa A, Stagner J L, Samols E. Supressive role of the islet-acinar axis in the perfused rat pancreas. Gastroenterology 1993;105:S868-887.
- Scymour N E, Volpan A H, Andersen U K, Hernandez C. Alteration in hepatocyte insulin binding in chronic pancreatitis: efrects of pancreatic polypeptide. Amer J of Surgery1995;169:105-110.
- Patto R J, Russo E K, Borges D, Neves M. The entero-insular axis and endocrine pancreatic function in chronic alcoholic consumers: evidence for early beta-cell hypofunction. Mt Sinai J Med 1993;60(4):317-320.
Correspondencia: Osvaldo Manuel Tiscornia
JE Uriburu 1044 PB 7, Ciudad Autónoma de Buenos Aires, Argentina
E-mail: doctis27@gmail.com, otero.graciela@gmail.com, fabiannen@gmail.com
Acta Gastroenterol Latinoam 2015;45:155-164
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE