Víctor Hugo Bardales–Zuta1,2 ID· Sandra Reyes–Aroca2,3 ID· Miguel de los Santos Verona–Escurra2,3 ID· Heber Giancarlo Moya-Carranza2 ID· Lissett Jeanette Fernández – Rodríguez2,4 ID
1 Internal Medicine Service, Hospital I Florencia de Mora EsSalud, Florencia de Mora, La Libertad, Peru.
2 School of Medicine, Universidad Privada Antenor Orrego, Trujillo, La Libertad, Peru.
3 Gastroenterology Service, Hospital de Alta Complejidad Virgen de la Puerta EsSalud, La Esperanza, La Libertad, Peru.
4 Oncology Deparment, Hospital Regional Lambayeque, Chiclayo, Lambayeque, Peru.
Acta Gastroenterol Latinoam 2024;54(3):239-246
Recibido: 29/07/2024 / Aceptado: 24/09/2024 / Publicado online el 30/09/2024 /
https://doi.org/10.52787/agl.v54i3.428
Summary
Objective. Although the causes of colorectal adenoma have been well characterized in other populations, this study is the first to investigate the risk factors for colorectal adenoma in a Peruvian population. Material and Methods. This is an observational, retrospective, case-control study of patients who underwent colonoscopy at the gastroenterology service of a large hospital in northern Peru between 2015 and 2020. Two groups of 138 patients were selected based on colorectal adenoma diagnosis. Gender, age, and the presence of non-alcoholic fatty liver disease, diabetes, obesity, hypertension and dyslipidemia were compared between groups to calculate risk factors for colorectal adenoma. These are known risk factors in other populations. Results. Among the measured factors, non-alcoholic fatty liver disease and male sex were found to be associated with colorectal adenoma (OR 3.3, 95% CI Interval 1.8-6.1 for non-alcoholic fatty liver disease, and OR 2.2 95% CI 1.3-3.6 for male sex). Other socio-medical characteristics did not reach statistical significance. Furthermore, no significant differences in location, number, size, endoscopic classification, histology or presence of advanced adenoma were observed when comparing patients diagnosed with non-alcoholic fatty liver disease with patients without this condition. Conclusion. This study suggests that non-alcoholic fatty liver disease and male sex are positively associated with the diagnosis of colorectal adenoma in Peruvians. This indicates the need for more careful screening of these demographics.
Keywords. Nonalcoholic fatty liver disease, colorectal adenoma, risk factor, Peru.
La enfermedad del hígado graso no alcohólico y el sexo masculino son factores de riesgo para el adenoma colorrectal: un análisis retrospectivo
Resumen
Objetivo. Aunque las causas del adenoma colorrectal han sido bien caracterizadas en otras poblaciones, este estudio es el primero en investigar los factores de riesgo de adenoma colorrectal en una población peruana. Materiales y métodos. Se trata de un estudio observacional, retrospectivo, de casos y controles de pacientes a los que se realizaron colonoscopias en el servicio de gastroenterología de un gran hospital del norte de Perú entre 2015 y 2020. Se seleccionaron dos grupos de 138 pacientes según el diagnóstico de adenoma colorrectal. Se compararon el género, la edad y la presencia de enfermedad del hígado graso no alcohólico, diabetes, obesidad, hipertensión y dislipidemia entre los grupos para calcular los factores de riesgo de adenoma colorrectal. Estos son factores de riesgo conocidos en otras poblaciones. Resultados. De los factores medidos, se encontró que la enfermedad del hígado graso no alcohólico y el sexo masculino estaban asociados con el adenoma colorrectal (OR 3,3 IC 95% 1,8-6,1 para la enfermedad del hígado graso no alcohólico y OR 2,2 IC 95% 1,3-3,6 para el sexo masculino). Otras características sociomédicas no alcanzaron significancia estadística. Además, no se observaron diferencias significativas entre cuanto a la ubicación, número, tamaño, clasificación endoscópica, histología o presencia de adenoma avanzado al comparar los pacientes con diagnóstico de enfermedad del hígado graso no alcohólico con pacientes sin esa condición. Conclusión. Este estudio sugiere que la enfermedad del hígado graso no alcohólico y el sexo masculino están asociados positivamente con el diagnóstico de adenoma colorrectal entre los peruanos. Esto sugiere la necesidad de un tamizaje más cuidadoso de estos grupos demográficos.
Palabras claves. Enfermedad del hígado graso no alcohólico, adenoma colorrectal, factor de riesgo, Perú.
Abbreviations
Colorectal cancer: CRC.
Colorectal adenoma: CRA.
Non-alcoholic fatty liver disease: NAFLD.
Hospital de Alta Complejidad Virgen de la Puerta: HACVP.
Glycated Hemoglobin: HbA1c.
Interquartile range: IQR.
Odds ratios: OR.
95% confidence interval: 95% CI.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death and the second most common malignancy in the world. In Peru, CRC has the fourth highest incidence and mortality among cancers.1 CRC develops in the colon and rectal epithelium through the accumulation of genetic alterations that begin as colorectal adenomas (CRAs) before becoming malignant.2 Therefore, early detection and removal of CRA through colonoscopy has been shown to be the most effective way to reduce the incidence, morbidity, and mortality of CRC.3,4
There are many factors that modify the risk of CRC. Some well-characterized factors that have been shown to increase the risk of CRC include age, male sex, physical inactivity, obesity, family history, metabolic syndrome, insulin resistance, tobacco smoking, alcohol consumption, radiation exposure, immunosuppression, race, and a diet high in processed red meat. On the other hand, physical activity and a high fiber diet rich in fruits, vegetables, and fish are associated with lower risk. These factors have varying levels of risk and quality of evidence.5-14 Many of the same risk factors associated with CRC are also associated with the development of CRA.15–19
Another well-established independent risk factor for CRC and CRA is non-alcoholic fatty liver disease (NALFD).5,10,12,17,18,20-36 NALFD is characterized by hepatic steatosis and, if allowed to progress, can lead to fibrosis, cirrhosis, and hepatic and extra-hepatic cancers.36 It is currently the most common liver disease worldwide and its prevalence is above average in Latin America.37 It causes overlap with those of CRC. It is associated with obesity, metabolic syndrome, diabetes, age, male sex, and genetics.20,37-39
Sociodemographic factors associated with NALFD, CRC, and CRA also tend to increase with economic development, which makes more food available to a larger population and encourages sedentary lifestyles. This trend is evident in Peru, where obesity rates have risen steadily over the last 30 years in tandem with economic growth.40 Because the risk factors for CRA have been identified in studies conducted primarily in Asia and North America, it is not entirely clear that these findings can be generalized to the rest of the world. Thus, additional studies in other populations are needed to confirm these findings in additional populations or to demonstrate that CRA risk factors differ by race, ethnicity, or other population labels.
Therefore, to better understand the risk factors for CRA in a Peruvian population, we conducted an observational, retrospective case-control study to determine whether NAFLD and other sociodemographic factors such as sex, age, hypertension, dyslipidemia, and obesity are independent risk factors for CRA. This information would help develop region-specific strategies to better focus resources for the treatment and screening of CRA in Peru, with the aim of detecting CRA before it becomes malignant.
Materials and Methods
Demographics
This is an analytical retrospective case-control study of patients seen at the gastroenterology service of the Hospital de Alta Complejidad Virgen de la Puerta (HACVP) in La Esperanza, La Libertad, Peru, between 2015 and 2020. This study was approved by the ethical review boards of EsSalud, the parent institution of HACVP and Universidad Privada Antenor Orrego, the sponsoring institution. The approval resolution codes were CONSTANCIA N°154 ESSALUD NIT: 9070-2023-1866 and N°0137-2023-UPAO respectively. Since this study involved only a review of medical records collected during routine treatment and no identifiable information was collected, informed consent was not required.
Inclusion Criteria
Medical records of patients older than 50 years who underwent abdominal echography or tomography prior to routine colonoscopy screening were considered for inclusion. Reasons for routine colonoscopy screening included CRC screening, presence of gastrointestinal symptoms, follow-up after resection of adenomatous polyps, family history of CRC, and positive fecal occult blood test. To be included in the study, medical records must include evidence of adequate colonic preparation and a complete colonoscopy. Adequate colonic preparation required a clear liquid diet for 48 hours prior to colonoscopy, ingestion of 4 L of polyethylene glycol solution 24 hours prior to colonoscopy, and a an 8-hour fast prior to colonoscopy. The colon must have had a Boston bowel preparation score of 2 or more for the right, transverse, and left colon, with a minimum total score of 6.41 Records of complete colonoscopies that reached the cecum were included. This was confirmed by visualization of the appendicular orifice, ileocecal valve, and convergence of the taenia coli.
Records with incomplete or missing clinical history, colonic preparation, or colonoscopy as defined above were excluded. Other reasons for exclusion included records of heavy alcohol consumption (> 210 g/week for men, > 140 g/week for women), history of colon diseases such as CRC or inflammatory bowel disease, history of liver diseases such as hepatocellular carcinoma, cirrhosis, viral hepatitis, presence of autoimmune diseases such as primary biliary cirrhosis, history of metabolic diseases such as Wilson’s disease, hemochromatosis or cystic fibrosis, or use of steatogenic drugs such as tamoxifen, amiodarone, methotrexate, or valproic acid.
Variables and Measurements
For each record that met the selection criteria for this study, age, sex and the presence of existing health conditions (hypertension, dyslipidemia, diabetes, obesity and / or NAFLD) were recorded. Hypertension was defined as systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg measured at separate visits.42 Diabetes was defined as fasting glucose ≥ 126 mg/dL, HbA1c ≥ 6.5%, plasma glucose ≥ 200 mg/dL after ingestion of a 75 g glucose bolus, and/or the presence of classic symptoms.43 Dyslipidemia was defined as cholesterol ≥ 200 mg/dL, triglycerides ≥ 150 mg/dL, LDL ≥ 100 mg/dL, and HDL ≤ 40 mg/dL in men and ≤ 50 mg/dL in women, or a history of lipid-lowering treatment.44 Obesity was defined as a body mass index (BMI) of ≥ 30 kg/m2.45 A patient had NAFLD if the diagnosis was made by a HACVP radiologist interpreting abdominal ultrasound or tomography. HACVP-positive findings included diffuse increase in liver echogenicity, greater liver echogenicity compared to kidney and spleen, and/or little or no visualization of the walls of the intrahepatic vessels, diaphragm, and posterior portion of the right hepatic lobe.
All colonoscopies were performed by experienced gastroenterologists from the gastroenterology service of the HACVP using Olympus® 160 colonoscopes. Each colonoscopy took a minimum of 9 minutes. The procedures were performed under sedation administered by a specialized nurse. Preparation quality was reported using the Boston scale. Colonoscopy results will also be recorded, including location, number, endoscopic classification, and histology results of resected adenomas, as well as the degree of dysplasia and presence of advanced adenoma (size greater than 10 mm with presence of histopathologic components of villous or tubulovillous or high-grade dysplasia).
For this study, cases were defined as patients with colonoscopy results of histopathologically confirmed colorectal adenomatous polyps. Controls were defined as patients with normal colonoscopy results.
Study Size
Study size calculations were performed using Epidat v 3.0, estimating a 95% confidence interval, 80% power, and a case to control ratio of 1:1. Results from a previous study23 were used to estimate the proportion of NAFLD+ and CRA+ (cases, 55.6%) and the proportion of NAFLD- and CRA+ (controls, 38.8%). This calculation estimated a study size of 276 patients with two groups of 138 patients each. Probabilistic simple random sampling was used to select records that met the selection criteria.
Statistical Analysis
IBM SPSS v. 26.0 was used for statistical calculations. Frequencies and percentages were calculated from the recorded data. Statistical tests included descriptive statistics (means, medians, ranges, proportions, and standard deviation) as appropriate. Age was expressed as median and interquartile range (IQR); categorical variables were expressed as proportions. For bivariate analysis, a Student T or Mann-Whitney U test was used for age, and chi-square (χ2) for the other categorical variables. Associations were considered significant when p < 0.05. Odds ratios (OR) were calculated with 95% confidence intervals. Similarly, multivariate analysis was performed by logistic regression of the intervening variables.
Results
After estimating and selecting the study size, two groups of 138 patient records that met the inclusion criteria were created by randomly sampling the medical records of patients seen at the HACVP between 2015 and 2020. Five main reasons for requesting routine colonoscopy were identified: screening for CRC (55%), gastrointestinal symptoms (23%), follow-up after resection of adenomatous polyps (8%), family history of colorectal cancer (12%) and positive fecal occult blood test (2%). Boston bowel preparation scores were not different between the case and control groups.
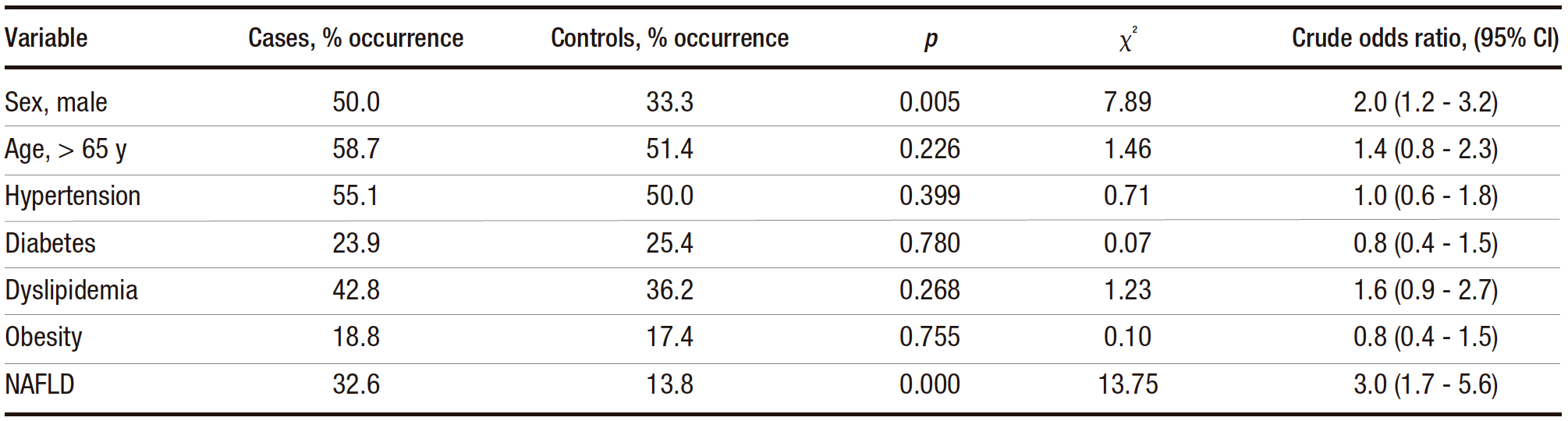
The median age was 69 (IQR: 59-76) and 67 (IQR: 57-74) years in the case and control groups, respectively. The result of the Mann-Whitney U test for age did not reach statistical significance (p = 0.156), indicating that age was not a significant factor in the development of CRA. Categorical variables were subjected to bivariate analysis (Table 1). The results of the statistical tests indicated that both male sex and the presence of NAFLD were risk factors for CRA, with NAFLD having a higher odds ratio. The other variables did not appear to be risk factors for CRA.
Table 1. Distribution of cases and controls for categorical variables tested for association with CRA (chi-square analysis)
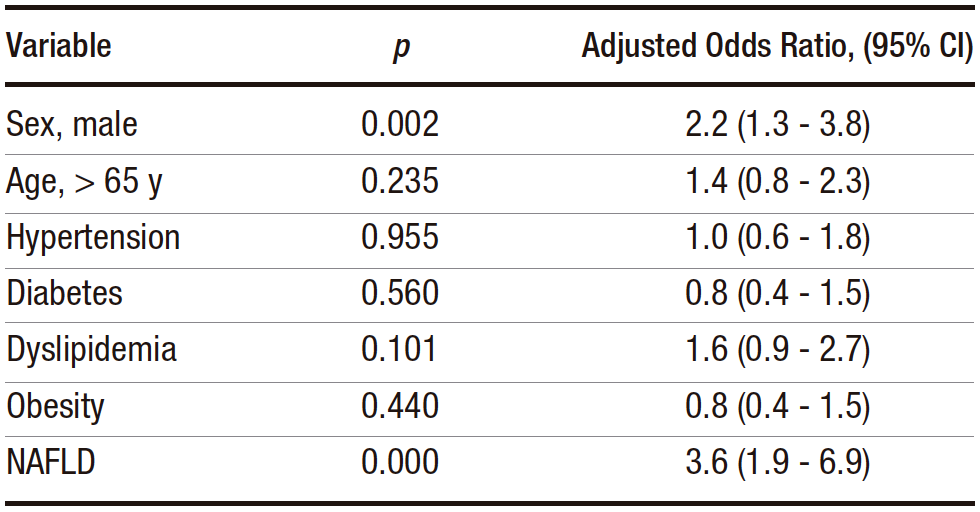
To observe whether sex and NAFLD varied together, a multivariate analysis was performed (Table 2), which showed that NAFLD and male sex remained independent risk factors for CRA. When adjusted for male sex, NAFLD remained a risk factor for CRA (adjusted odds ratio 3.3, 95% CI 1.8-6.1, p < 0.000). Similarly, male sex remained a risk factor after adjustment for NAFLD (adjusted odds ratio 2.2, 1.3-3.6).
Table 2. Results of the multivariate analysis with adjusted odds ratios and confidence intervals for the factors tested for association with CRA
The results were also analyzed to see if NAFLD was associated with different colonoscopy findings. In this analysis, patient records were grouped according to whether the patients were diagnosed with NAFLD or not, and the proportions of colonoscopy findings were analyzed by chi-square analysis (Table 3). Of the records included, 45 patients had NAFLD and 93 did not. Statistical analysis failed to find an association between the diagnosis of NAFLD and any specific colonoscopy finding, although polyp classification was close to the significance level.
Table 3. Colonoscopy findings related to NAFLD analyzed by Chi-square. The results are presented as percentages

Discussion
Most studies analyzing risk factors for CRA have been conducted in Asia, with some others in Europe and North America.12,15-33 Since this is the first study of its kind in Peruvian patients, it is possible to compare risk factors between different populations. We find that NAFLD is a risk factor for CRA in Peruvian patients, with an odds ratio of approximately 3. When controlling for male sex, NAFLD remains a risk factor for CRA and vice versa. Other factors studied, such as age, hypertension, diabetes, dyslipidemia, and obesity, did not show an association with CRA. In addition, the presence of NAFLD is not associated with specific colonoscopy findings, only with the presence or absence of CRA.
Our finding that NAFLD is a risk factor for CRA is supported by the literature, but the odds ratio and confidence interval calculated here are higher than almost all but one published studies. Approximately half of the confidence intervals overlap in the studies we were able to find and compare.11,22-27,29,30,32,33,46,47 Given this, the risk of developing CRA after a diagnosis of NAFLD in this population appears to be higher than previously estimated. Regardless of the magnitude of the risk, patients with NAFLD are at a higher risk of CRA than the general population in many parts of the world.
Previous studies has been helpful in proposing mechanisms for the NAFLD-CRA association. There are likely four main mechanisms that mediate this association: insulin resistance, chronic inflammation, adopokines, and alterated intestinal microecology. Insulin resistance, often associated with NAFLD, sets the stage for CRA by causing hyperinsulinemia, which can directly stimulate neoplastic growth of the colonic mucosa and indirectly promote the development of colorectal tumors by increasing IGF-1 levels. The release of pro-inflammatory cytokines (IL-6 and TNF-α) in patients with NAFLD can further stimulate this process. Patients with NAFLD also have low plasma levels of adiponectin, which has anti-inflammatory and anti-apoptotic effects and increases insulin sensitivity. In addition, leptin levels are elevated in patients with NAFLD, which has a carcinogenic effect. Finally, intestinal dysbiosis increases intestinal permeability to bacterial metabolites, causing inflammation and liver damage, accelerating a chronic systemic inflammatory state, and producing genotoxins that interfere with intestinal cell cycle regulation.20,21,30,31
We also find that CRA and male sex are independently associated with an odds ratio of approximately 2. This finding also has strong support in the literature, and has been linked to gender behaviors, sex hormones, sex differences in immune behaviors, and differences in the microbiome between both sex.28,31-33,48
The majority of the CRA patients in this study were over 65 years of age. Although not reaching statistical significance in this study, several other reports conclude that age is a risk for CRA.9,14,26 A likely reason is that time allows for the accumulation of mutations that lead to CRA.
Our study did not show that hypertension, diabetes, dyslipidemia, and obesity were risk factors for CRA in this population; however, these factors are sometimes mentioned as being associated with CRA. For example, KW Huang et al.23 found that high blood pressure and smoking were independent risk factors for CRA after a negative baseline colonoscopy, while no significant association with diabetes was found. However, studies by ST Hwang et al.47 and Liu M et al.27 show that arterial hypertension and diabetes were not associated with colorectal adenomas, but tobacco smoking was.
Furthermore, ST Hwang et al.47 found that metabolic syndrome, increased abdominal circumference and hypertriglyceridemia were associated with CRA. At the same time, Li Y. et al.26 demonstrated that higher mean fasting plasma glucose, BMI, and high triglyceride levels were risk factors for CRA. Similarly, the meta-analysis by Ding, W et al.34 it was found that patients with CRA had a statistically significant difference in BMI, abdominal circumference, triglycerides, HDL and fasting glucose compared to patients without CRA. Likewise, hypertriglyceridemia, low HDL level, BMI ≥ 25 and fasting glucose intolerance (≥ 100 mg/dL) were risk factors for the development of colorectal adenomas.
These different results may be explained by differences in study design or differences in study populations. This study may have defined the presence of diabetes or dyslipidemia slightly differently and did not classify these factors into subtypes. For example, average lipid profiles were not considered in this study. In the same way, abdominal circumference, central obesity, tobacco smoking, and metabolic syndrome were not included. Given these differences, it is also possible that there are unique characteristics of the study population that exclude some risk factors seen in other studies.
As an additional analysis, colonoscopy findings were evaluated to determine if any were associated with NAFLD: No statistically significant differences were found (Table 3). Shen et al.49 found that NAFLD was only significantly associated with the number of colorectal adenomas (≥ 3), but not with the location, size, or presence of advanced adenomas. Regarding location, the meta-analysis by Lin et al.35 established that the pooled total risk value was higher for right (proximal) colon tumors than for left (distal) colon tumors in Asian populations, whereas the risk was similar, but slightly higher for bilateral colorectal tumors in the European and American populations. This is consistent with the present study, where the incidence of CRA was similar in the distal and proximal colon, regardless of NAFLD status. Furthermore, if only flexible sigmoidoscopy was used as a screening method in these patients, a large proportion of CRAs may have been missed.
Limitations
The first and probably most important limitation of this study is the way in which NAFLD was diagnosed from medical records. The gold standard for diagnosis is liver biopsy, but this is not a routine procedure in the HACVP, so ultrasound or tomography results were used instead, limiting diagnostic certainty and potentially introducing prevalence bias. Second, the data were collected retrospectively, which may have caused reporting or standardization bias. Also, confounding variables are difficult to account for and eliminate in a retrospective study.
Conclusion
For the first time, a retrospective case-control study was conducted in a Peruvian population to search for risk factors associated with CRA in other populations, showing that only the presence of NAFLD or male sex were significant independent risk factors. These results are likely to be generalizable to the Peruvian population and can likely inform CRA screening practices and largely confirm results found in other populations, although some risk factors reported elsewhere did not reach significance in this study. Based on these results, men and patients with NAFLD in Peru should be monitored more frequently with colonoscopy, but more detailed studies in similar populations are needed to confirm these findings.
Consent for Publication. Anonymized data were used for the elaboration of this article, which did not distort its scientific value.
Intellectual Property. The authors declare that the data and tables that appear in this article are original and were made in their belonging institutions.
Funding. The authors declare that there were no external sources of funding.
Conflict of Interest. The authors declare that they have no conflicts of interest related to this article.
Copyright
© 2024 Acta Gastroenterológica latinoamericana. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license, which allows non-commercial use, distribution, and reproduction, provided the original author and source are acknowledged.
Cite this article as: Bardales-Zuta V H, Reyes-Aroca S, de los Santos Verona-Escurra M et al. Nonalcoholic Fatty Liver Disease and Male Sex are Risk Factors for Colorectal Adenoma: a Retrospective Analysis. 2024;54(3):239-246. https://doi.org/10.52787/agl.v54i3.428
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021;71(3):209-49. Available from: https://doi.org/10.3322/caac.21660
- Nguyen LH, Goel A, Chung DC. Pathways of Colorectal Carcinogenesis. Gastroenterology. 2020 Jan;158(2):291-302. Available from: https://doi.org/10.1053/j.gastro.2019.08.059
- Galeș LN, Păun MA, Anghel RM, Trifă nescu OG. Cancer Screening: Present Recommendations, the Development of Multi-Cancer Early Development Tests, and the Prospect of Universal Cancer Screening. Cancers (Basel). 2024 Mar 18;16(6):1191. Available from: https://doi.org/10.3390/cancers16061191
- Areia M, Spaander MC, Kuipers EJ, Dinis-Ribeiro M. Endoscopic screening for gastric cancer: A cost-utility analysis for countries with an intermediate gastric cancer risk. United European Gastroenterol J. 2018 Mar;6(2):192-202. Available from: https://doi.org/10.1177/2050640617722902
- Steck SE, Guinter M, Zheng J, Thomson CA. Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv Nutr. 2015 Nov;6(6):763-73. Available from: https://doi.org/10.3945/an.115.009746
- Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002 Dec;31(4):925-43. Available from: https://doi.org/10.1016/s0889-8553(02)00057-2
- Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010 Jun;138(6):2044-58. Available from: https://doi.org/10.1053/j.gastro.2010.01.054
- O’Sullivan DE, Sutherland RL, Town S, Chow K, Fan J, Forbes N, et al. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clinical Gastroenterology and Hepatology. 2022 Jun 1;20(6):1229-1240.e5. Available from: https://doi.org/10.1016/j.cgh.2021.01.037
- Sninsky JA, Shore BM, Lupu GV, Crockett SD. Risk Factors for Colorectal Polyps and Cancer. GastrointestEndosc Clin N Am. 2022 Apr;32(2):195-213. Available from: https://doi.org/10.1016/j.giec.2021.12.008
- Sun L, Yu S. Diabetes Mellitus Is an Independent Risk Factor for Colorectal Cancer. Dig Dis Sci. 2012 Jun 1;57(6):1586-97. Available from: https://doi.org/10.1007/s10620-012-2059-x
- Shen X, Wang Y, Zhao R, Wan Q, Wu Y, Zhao L, et al. Metabolic syndrome and the risk of colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021 Oct 1;36(10):2215-25. Available from: https://doi.org/10.1007/s00384-021-03974-y
- Liu SS, Ma XF, Zhao J, Du SX, Zhang J, Dong MZ, et al. Association between nonalcoholic fatty liver disease and extrahepatic cancers: a systematic review and meta-analysis. Lipids in Health and Disease [Internet]. 2020 May 31 [cited 2024 May 30];19(1):118. Available from: https://doi.org/10.1186/s12944020-01288-6
- Grahn SW, Varma MG. Factors that Increase Risk of Colon Polyps. Clin Colon Rectal Surg [Internet]. 2008 Nov;21(4):247-55. Available from: https://doi.org/10.1055/s-0028-1089939
- Menon G, Recio-Boiles A, Lotfollahzadeh S, Cagir B. Colon Cancer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 May 31]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK470380/
- He X, Wu K, Ogino S, Giovannucci EL, Chan AT, Song M. Association Between Risk Factors for Colorectal Cancer and Risk of Serrated Polyps and Conventional Adenomas. Gastroenterology. 2018 Aug;155(2):355-373.e18. Available from: https://doi.org/10.1053/j.gastro.2019.06.039
- Wu H, Zhang J, Zhou B. Metabolic syndrome and colorectal adenoma risk: A systematic review and meta‑analysis. Clin Res Hepatol Gastroenterol. 2021 Sep;45(5):101749. Available from: https://doi.org/10.1016/j.clinre.2021.101749
- Okabayashi K, Ashrafian H, Hasegawa H, Yoo JH, Patel VM, Harling L, et al. Body Mass Index Category as a Risk Factor for Colorectal Adenomas: A Systematic Review and Meta-Analysis. Official journal of the American College of Gastroenterology | ACG. 2012 Aug;107(8):1175. Available from: https://doi.org/10.1038/ajg.2012.180
- Tian Y, Wang K, Li J, Wang J, Wang Z, Fan Y, et al. The association between serum lipids and colorectal neoplasm: a systemic review and meta-analysis. Public Health Nutrition. 2015 Dec;18(18):3355-70. Available from: https://doi.org/10.1017/s1368980015000646
- Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet. 2016 Dec;29(6):757-67. Available from: https://doi.org/10.1111/jhn.12395
- Manikat R, Nguyen MH. Nonalcoholic fatty liver disease and non-liver comorbidities. Clin Mol Hepatol. 2023 Jan 5;29(Suppl):s86-102. Available from: https://doi.org/10.3350/cmh.2022.0442
- Parizadeh SM, Parizadeh SA, Alizade-Noghani M, Jafarzadeh-Esfehani R, Ghandehari M, Mottaghi-Moghaddam A, et al. Association between non-alcoholic fatty liver disease and colorectal cancer. Expert Review of Gastroenterology & Hepatology. 2019 Jul 3;13(7):633-41. Available from: https://doi.org/10.1080/17474124.2019.1617696
- Wu PH, Chung CH, Wang YH, Hu JM, Chien WC, Cheng YC. Association between nonalcoholic fatty liver disease and colorectal cancer: A population-based study. Medicine. 2023 May 26;102(21):e33867. Available from: https://doi.org/10.1097/md.0000000000033867
- Huang KW, Leu HB, Wang YJ, Luo JC, Lin HC, Lee FY, et al. Patients with nonalcoholic fatty liver disease have higher risk of colorectal adenoma after negative baseline colonoscopy. Colorectal Disease. 2013;15(7):830-5. Available from: https://doi.org/10.1111/codi.12172
- Blackett JW, Verna EC, Lebwohl B. Increased Prevalence of Colorectal Adenomas in Patients with Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study. Dig Dis. 2020;38(3):222-30. Available from: https://doi.org/10.1159/000502684
- Chen J, Bian D, Zang S, Yang Z, Tian G, Luo Y, et al. The association between nonalcoholic fatty liver disease and risk of colorectal adenoma and cancer incident and recurrence: a meta-analysis of observational studies. Expert Review of Gastroenterology & Hepatology. 2019 Apr 3;13(4):385-95. Available from: https://doi.org/10.1080/17474124.2019.1580143
- Li Y, Liu S, Gao Y, Ma H, Zhan S, Yang Y, et al. Association between NAFLD and Risk of Colorectal Adenoma in Chinese Han Population. Journal of Clinical and Translational Hepatology. 2019 Jun 28;7(2):99-105. Available from: https://doi.org/10.14218/jcth.2019.00010
- Liu M, Dai F, Peng Q. Relationship between severity of liver fibrosis and colorectal adenomatous polyp in nonalcoholic fatty liver disease. Chin J IntegrTradit West Med Dig. 2022 Jan 15;30(1):36-41. Available from: https://zxyxhen.whuhzzs.com/article/doi/10.3969/j.issn.1671-038X.2022.01.08
- Chuan LX, Chang J, Zhao JH, Li LH, Yang XY, Yu D. Association between nonalcoholic fatty liver disease and colorectal adenomatous polyps. J Clin Hepatol. 2020 Jun 20;36(6):1299-303. Available from: https://www.lcgdbzz.org/en/article/doi/10.3969/j.issn.1001-5256.2020.06.022
- Ye S, Liu Y, Zhang T, Feng H, Liu Y, Ma L. Analysis of the correlation between non-alcoholic fatty liver disease and the risk of colorectal neoplasms. Front Pharmacol. 2022 Nov 9;13. Available from: https://doi.org/10.3389/fphar.2022.1068432
- Lee JM, Park YM, Yun JS, Ahn YB, Lee KM, Kim DB, et al. The association between nonalcoholic fatty liver disease and esophageal, stomach, or colorectal cancer: National population-based cohort study. PLOS ONE. 2020 Jan 24;15(1):e0226351. Available from: https://doi.org/10.1371/journal.pone.0226351
- Chen W, Wang M, Jing X, Wu C, Zeng Y, Peng J, et al. High risk of colorectal polyps in men with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Journal of Gastroenterology and Hepatology. 2020;35(12):2051-65. Available from: https://doi.org/10.1111/jgh.15158
- Pan S, Hong W, Wu W, Chen Q, Zhao Q, Wu J, et al. The relationship of nonalcoholic fatty liver disease and metabolic syndrome for colonoscopy colorectal neoplasm. Medicine. 2017 Jan;96(2):e5809. Available from: https://doi.org/10.1097/md.0000000000005809
- Chen QF, Zhou XD, Sun YJ, Fang DH, Zhao Q, Huang JH, et al. Sex-influenced association of non-alcoholic fatty liver disease with colorectal adenomatous and hyperplastic polyps. World Journal of Gastroenterology. 2017 Jul 28;23(28):5206-15. Available from: https://doi.org/10.3748/wjg.v23.i28.5206
- Ding W, Fan J, Qin J. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(1):322-33. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4358458/
- Lin X, You F, Liu H, Fang Y, Jin S, Wang Q. Site-specific risk of colorectal neoplasms in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. PLOS ONE. 2021 Jan 25;16(1):e0245921. Available from: https://doi.org/10.1371/journal.pone.0245921
- Mitsala A, Tsalikidis C, Romanidis K, Pitiakoudis M. Non-Alcoholic Fatty Liver Disease and Extrahepatic Cancers: A Wolf in Sheep’s Clothing? Curr Oncol. 2022 Jun 25;29(7):4478-510. Available from: https://doi.org/10.3390/curroncol29070356
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 Jul;64(1):73-84. Available from: https://doi.org/10.1002/hep.28431
- Marjot T, Moolla A, Cobbold JF, Hodson L, Tomlinson JW. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocrine Reviews. 2020 Feb 1;41(1):66-117. Available from: https://doi.org/10.1210/endrev/bnz009
- Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC EndocrDisord. 2022 Mar 14;22:63. Available from: https://doi.org/10.1186/s12902-022-00980-1
- Risk Factor Collaboration. NCD-RisC [Internet]. [cited 2024 May 26]. Available from: https://ncdrisc.org/data-downloads-adiposity.html
- Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. GastrointestEndosc. 2009 Mar;
69(3 Pt 2):620-5. Available from: https://doi.org/10.1016/j.gie.2008.05.057 - Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension [Internet]. 2020 Jun [cited 2024 May 29];75(6):1334-57. Available from: https://www.doi.org/10.1161/HYPERTENSIONAHA.120.15026
- ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care [Internet]. 2022 Dec 12 [cited 2024 May 29];46(Supplement_1):S19-40. Available from: https://doi.org/10.2337/dc23-S002
- Pappan N, Awosika AO, Rehman A. Dyslipidemia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 May 29]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK560891/
- Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016 Jun;22(7 Suppl):s176-185. Available from: https://www.ajmc.com/view/obesity-definition-comorbidities-causes-burden
- Wong VWS, Wong GLH, Tsang SWC, Fan T, Chu WCW, Woo J, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut. 2011 Jun;60(6):829-36. Available from: https://doi.org/10.1136/gut.2011.237974
- Hwang ST, Cho YK, Park JH, Kim HJ, Park DI, Sohn CI, et al. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. Journal of Gastroenterology and Hepatology. 2010;25(3):562-7. Available from: https://doi.org/10.1111/j.1440-1746.2009.06117.x
- Choi Y, Kim N. Sex Difference of Colon Adenoma Pathway and Colorectal Carcinogenesis. World J Mens Health. 2024 Apr;42(2):256-82. Available from: https://doi.org/10.5534%2Fwjmh.230085
- Shen H, Lipka S, Kumar A, Mustacchia P. Association between nonalcoholic fatty liver disease and colorectal adenoma: a systemic review and meta-analysis. Journal of Gastrointestinal Oncology. 2014 Dec;5(6). Available from: https://doi.org/10.3978%2Fj.issn.2078-6891.2014.061
Correspondence: Víctor Hugo Bardales-Zuta
Mail: vbardalesz@upao.edu.pe
Acta Gastroenterol Latinoam 2024;54(3):239-246
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE


