Lucy Bravo-Luna1 ID· Rosa Amalia Dueñas-Cuellar2 ID· Victoria Eugenia Niño-Castaño2 ID Ángela Merchán-Galvis3 ID· Luisa Katherine Orozco-Morales4 ID· Cristhian Danilo Padilla-Medina4 ID· Harold Bolaños3 ID
1 Department of Pathology, Universidad del Cauca, Colombia.
2 Department of Pathology, Immunology and Infectious Diseases Research Group, School of Health Sciences, Universidad del Cauca, Colombia.
3 Department of Social Medicine and Family Health, Universidad del Cauca, Colombia.
4 Research Seedbed in Immunology and Infectious Diseases, School of Health Sciences, Universidad del Cauca, Colombia.
Acta Gastroenterol Latinoam 2022;52(4):484-493
Received: 19/04/2022 / Accepted: 28/11/2022 / Published online 21/12/2022 / https://doi.org/10.52787/agl.v52i4.200
Summary
Introduction. The Cauca region, in Colombia, has a high incidence of gastric cancer and there are few studies describing tumor microenvironment in gastric cancer samples obtained from this geographic region. Aim. The aim of this study was to describe the infiltration of CD3+, CD8+ T cells, the expression of programmed death-1 (PD-1) and programmed death ligand 1 (PD-L1), and Epstein-Barr virus infection in biopsies from Cauca patients with advanced intestinal-type gastric adenocarcinoma. Methodology. This is a descriptive cross-sectional study of 24 gastrectomy samples out of 48 samples analyzed. Expression of CD3, CD8, PD-1, and PD-L1 was analyzed using immunohistochemistry, and infection by Epstein-Barr virus was analyzed by Epstein-Barr virus-encoded small RNA in-situ hybridization (EBER-ISH) in gastric tissue samples on formalin-fixed, paraffin-embedded. Finally, the immunoscore and the Combined Positive Score were calculated.
Results. ITGA samples were 21.8% ± 13.6% and 14.8% ± 14.8% CD3 + and CD8 + positive respectively, 100% had low PD-1 expression, CPS score for PDL-1, 91.7% had <1 and 8.3% had >1. In EBER-ISH, 20.8% were positive, and in immunoscore, 16.7% had a score > 25%. Conclusion. This paper reports advanced ITGA cases with an atypical tumor microenvironment, compared to previously reported data. It is important to consider that the tumor microenvironment in gastric cancer is heterogeneous, which makes the analysis of microsatellite instability in this type of sample relevant.
Keywords. Stomach neoplasms, programmed cell death 1 ligand 2 protein, Epstein-Barr virus, Herpesvirus 4 Human, CD8-Positive T-Lymphocytes.
Estudio descriptivo del microambiente inmunológico tumoral e infección por el virus de Epstein-Barr en el adenocarcinoma gástrico
Resumen
Introducción. La región del Cauca, Colombia, tiene una alta incidencia de cáncer gástrico y son pocos los estudios que describen el microambiente tumoral en muestras de cáncer gástrico de esta región geográfica. Objetivo. El objetivo del estudio fue describir la infiltración de células T CD3 + CD8+, la expresión de muerte programada-1 (PD-1) y ligando de muerte programada 1 (PD-L1), y la infección por el virus de Epstein-Barr en biopsias de adenocarcinoma gástrico tipo intestinal avanzado de pacientes de dicha región. Metodología. Se trata de un estudio descriptivo transversal realizado en 24 muestras de gastrectomía de las 48 analizadas. La expresión de CD3, CD8, PD-1 y PD-L1 se analizó por inmunohistoquímica y la infección por virus de Epstein-Barr mediante hibridación in-situ de pequeños ARN codificados por el virus de Epstein-Barr (EBER-ISH) en muestras de tejido gástrico fijadas e incluidas en parafina. Finalmente, se calcularon el inmunoscore y el puntaje combinado positivo. Resultados. Las muestras de adenocarcinoma gástrico tipo intestinal avanzado tenían 21,8% ± 13,6% y 14,8% ± 14,8% de CD3+ y CD8+ positivo respectivamente, el 100% tenía baja expresión de PD-1, en puntuación combinado positivo para PDL- 1, el 91,7% tenía < 1 y el 8,3% tenía > 1. En EBER-ISH el 20,8% dio positivo y en inmunoscore el 16,7% tenía un puntaje > 25%. Conclusión. Este trabajo reporta casos de adenocarcinoma gástrico tipo intestinal avanzado avanzados con microambiente tumoral atípico, comparado con lo reportado previamente. Es importante considerar que el microambiente tumoral en cáncer gástrico es heterogéneo, por lo que hace relevante el análisis de inestabilidad de microsatélites en este tipo de muestras.
Palabras claves. Neoplasias de estómago, muerte celular programada 1 Ligando 2 Proteína, virus de Epstein-Barr, Herpesvirus 4 Humano, linfocitos T CD8-positivo.
Abbreviations
GC: Gastric cancer.
PD-1: Programmed death-1.
PD-L1: Programmed death ligand 1.
EBV: Epstein-Barr virus.
ITGA: Intestinal Type Gastric Adenocarcinoma.
EBER-ISH: Epstein-Barr virus-encoded small RNAs chromogenic in situ hybridization.
FFPE: Formalin-fixed, paraffin-embedded.
CPS: Combined Positive Score.
TGCA: The Cancer Genome Atlas.
WHO: World Health Organization.
EBV+: EBV Positive.
MSI: Microsatellite instability.
CIN: Chromosomal instability.
TILs: Tumor-infiltrating lymphocytes.
H&E: Hematoxylin and eosin.
IHC: Immunohistochemistry.
NCCN: National Comprehensive Cancer Network.
Introduction
Even though the incidence of gastric cancer (GC) has decreased in recent years,1 it remains one of the most frequent malignant neoplasms worldwide.2 According to GLOBOCAN 2020, it was the fifth type of cancer and the fourth cause of cancer death in the world.3 In Colombia, by 2020, GC ranked fourth in incidence and was the leading cause of cancer death3 in the Department of Cauca, Southwestern Colombia. Although there is no recent information on incidence, the adjusted mortality rate for 2014 was 15.9%, one of the highest in the country, and ranking third place nationally.4
This neoplasm occurs more frequently in men (2:1 ratio) and is strongly related to age (around 65 years old).2 Patients may be asymptomatic in the early stages and in advanced stages report non-specific symptoms. 90% of cases are sporadic and 10% are associated with genetic abnormalities. The main risk factors are infection by Helicobacter pylori and Epstein-Barr virus (EBV).5, 6
There are different classification systems of GC based on tumor cytology and the architectural distribution of neoplastic cells. The most used are those of Lauren and the World Health Organization (WHO). But these classifications do not take into account the characteristics of the tumor microenvironment and do not provide information on the molecular and genetic bases that promote carcinogenesis nor do they determine the different types of histopathological phenotypes.1 In 2014, The Cancer Genome Atlas (TGCA) reported the results of the GC genome and proteome study, a project that identified the molecular subtypes and deregulated pathways in this cancer.7 Four molecular subtypes were defined: 1. EBV Positive (EBV+), 2. Microsatellite instability (MSI), 3. Genomically stable, and 4. Chromosomal instability (CIN). This molecular profile revolutionized the understanding of the heterogeneous mechanisms underlying the pathogenesis of GC1, 8 defining the characteristics of each subtype, information that explains the different phenotypes, the variable response to treatment, and the clinical course of patients.8
The molecular subtypes of GC, EBV+, and MSI, are histopathologically characterized by significant inflammatory infiltration into the tumor microenvironment, composed predominantly of CD3+ and CD8+ cells, which varies according to the pathobiology of the tumor subtype and the expression of PD-L1.8 PD-L1 expression is associated with the evasion of the immune system in the tumor microenvironment, being this the mechanism used by neoplastic cells to evade the host’s immune response to cancer.9 The density of tumor-infiltrating lymphocytes (TILs) in GC has been evaluated using immunoscore estimation, which is a score that quantifies the CD3+ and CD8+ cells infiltration in the tumor microenvironment.10-12 These studies concluded that a high immunoscore is associated with increased disease-free survival and overall survival.11-14
The findings in the tumor microenvironment provide information on important aspects of tumorigenesis and its relationship to the prognosis and treatment of advanced stage GC patients.15 In some regions of Colombia the incidence of GC is high and diagnosis of cancer is made only through histopathological characteristics of the tumor cell, without considering the TILs and the expression of immune control molecules. Taking this into account, the aim of this work was to describe the tumor microenvironment on biopsies of advanced intestinal type adenocarcinoma from patients coming from an area with high incidence of GC and mortality in Colombia.
Methodology
A descriptive cross-sectional study was carried out. The study included gastrectomies performed between 2014 and 2016 on patients diagnosed with ITGA who have been classified with TNM stage II or III, and whose blocks of FFPE tissue specimens were in an adequate state of conservation. Patients with the following diagnosis were excluded: diffuse ITGA, I ITGA with TNM stage I or IV, immunosuppression or HIV infection, history of malignant neoplasm or autoimmune disease, history of chemotherapy, radiotherapy, or immunosuppressive treatment, and those whose FFPE blocks were deteriorated.
FFPE blocks were collected from the pathology laboratory of the Hospital Universitario San José, Popayán, Cauca, Colombia. For each case, the diagnosis was confirmed by two pathologists through the review of the slides stained with hematoxylin and eosin (H&E).
Ethical Considerations
The principles set out in the Declaration of Helsinki were followed. All tissue samples were coded anonymously, guaranteeing the confidentiality of patient information and the results obtained from the samples analyzed. The study was approved by the Ethics Committee from the Hospital Universitario San José (Approval Act Number 6 from May 19, 2016) and the Ethics Committee of the Universidad de Cauca (identification code 5095).
Immunohistochemistry (IHC)
The IHC was performed according to the manufacturer’s instructions. Briefly, specific antibodies were used to identify PD-L1 (Clone ZR3 Zeta Corporation, California USA), PD-1 (Clone EP239 Cell Marque, Darmstadt, Germany), CD3 (Clone MRQ-39 Cell Marque, Darmstadt, Germany), and CD8 (Clone SP16 Cell Marque, Darmstadt, Germany) proteins on ITGA tissue sections. The tissue sections were dewaxed at 60°C for 1 hour, rehydrated and incubated with 0.3% hydrogen peroxide for 5 minutes at room temperature, blocked with 10% BSA (bovine albumin serum) for 10 minutes at room temperature, and incubated using the primary antibody at 4°C for 30 minutes, and then chromogen was added at room temperature for 1 hour. Finally, contrast staining with hematoxylin was performed. Sections of pharyngeal tonsil tissue were used as control tissue. All sections were observed and photographed under the microscope (Leica Biosystems, Wetzlar, Germany).
IHC Evaluation
For the PD-L1 expression, the membrane immunostaining (partial or complete) was evaluated with ≥ 1+ scores on viable tumor cells, lymphocytes, and macrophages. The evaluation was conducted using the CPS. The positive group for PD-L1 was defined with a CPS score ≥ 1, the negative cases constituted a CPS score < 1, as determined in the National Comprehensive Cancer Network (NCCN) guidelines.16
In the expression of PD-1, the membrane immunostaining of TILs was evaluated. The percentage of immunoreactive cells was quantified, with criteria of high expression > 20%, and the intensity of staining was evaluated as: 0 = Negative, 1+ = Weak, and 2+ = Intermediate-Strong; Low Intensity: 0 to 1+ and High Intensity: 2+. The reference values for PD-1 and infiltration of CD3 and CD8+ were taken according to the work of Wen et al. 2017.17
In order to study the immune response, the sites with the highest density of peritumoral and intratumoral lymphocytes were selected according to the previous review of the reference slide stained with hematoxylin and eosin, and the determination of CD3+ and CD8+ lymphocytes using the immunohistochemical technique.
Reactivity for CD3+ and CD8+ was considered positive when immunostaining on the cell membrane occurred. The average percentage of 4 high-powered fields (40x) representative of the tumor process was visually quantified. It was determined as low infiltration an average percentage < 20%, and high infiltration ≥ 20%.17 The immunoscore was calculated averaging the percentage of the CD3+ and CD8+ infiltration, and it was defined as a Low immunoscore: 0-25% and a High immunoscore: ≥ 25% following the standardized values for immunoscore in colon cancer according to the work of Pagès et al. 2018.11
The IHC sections analyses were validated and verified by two pathologists who did not know the clinical-pathological data of the patients.
Colorimetric In Situ Hybridization
EBV infection in tumor cells was demonstrated with EBER-ISH technique, and automated Ventana (Benchmark XT) equipment using an EBER 1 DNP probe (Ventana, cat # 760-1209A, Tucson, AZ) and the ISH iView Blue Plus antiDNP detection system (Ventana, Tucson, AZ) was also used according to the manufacturer’s indications.
EBER-ISH Evaluation
EBV infection through EBER-ISH (INFORM EBER probe from Roche) was positive when nuclear staining was observed in more than 80% of the tumor cells.
Statistical Analysis
Standard statistical tests were used to analyze clinical data. Univariate analyses and a bivariate analysis between the EBER-ISH variable, and the clinical and pathological variables were performed. To compare the relationship between the variables, the Pearson chi-square statistic or Fisher exact statistic was used according to the number of categories of the variables, and the significance level was established at p < 0.005. Data were analyzed with the statistical software SPSS version.25
Results
A total of 48 clinical records of patients with ITGA who underwent gastrectomy at the Hospital Universitario San José in Popayán (2014 to 2016) were analyzed. The study population was determined applying the inclusion and exclusion criteria. 24 patients with ITGA were included in the study; 66.7% were men (n= 16) and 33.3% were female (n= 8). The mean age at diagnosis was 65 years old with a standard deviation of ± 12.8 years. All cases were histologically characterized as lymphoepithelioma-like carcinoma; moreover, in 22 cases, the TNM stage was III and in 2 cases, stage II. The histopathological characteristics are presented in Table 1.
Table 1. Percentage distribution of histopathological variables in samples of patients diagnosed with intestinal type gastric adenocarcinoma (2014 – 2016)
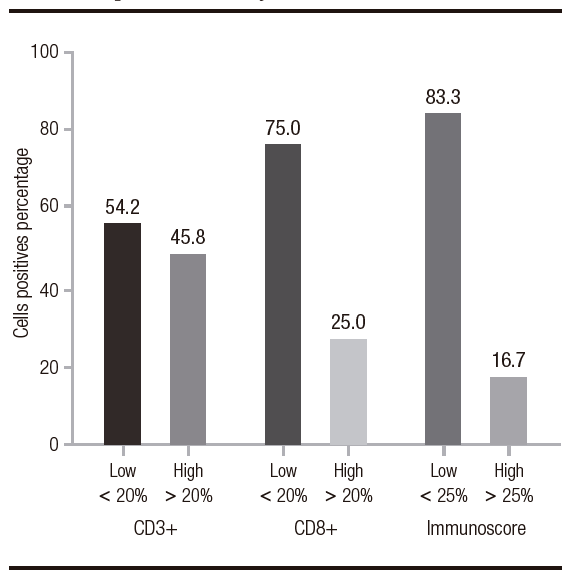
The percentage of the CD3+ infiltration averaged 21.8% (SD: 13.6%) and the CD8+ infiltration averaged 14.8% (SD: 10.9%). In the immunoscore calculation, the highest percentage was 62.5% and the lowest was 6%, with an average of 18.1% (SD: 12.2%). The percentage distribution is shown in Figure 1.
Figure 1. Percentage distribution of variables CD3+, CD8+ and immunoscore in samples of patients diagnosed with intestinal type gastric adenocarcinoma (2014-2016). Each bar represents mean of values
Intensity and Percentage of PD-1 Expression and Report of the CPS in Samples from Patients with Advanced ITGA
All ITGA samples had low expression of PD-1 (< 20%) and weak intensity of immunostaining. The calculation of CPS, in which the PD-L1 protein expression is reported, revealed that 91.7% of the cases (n= 22) had a score < 1 and only 8.3% (n= 2) had a score ≥ 1 (Figure 2).
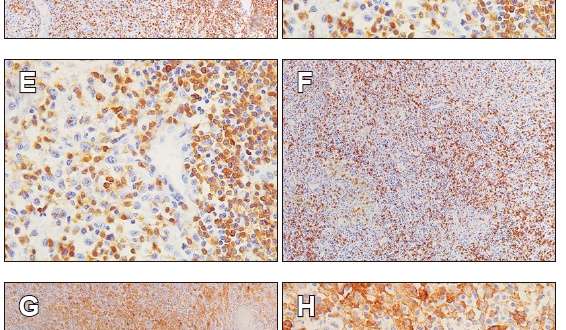
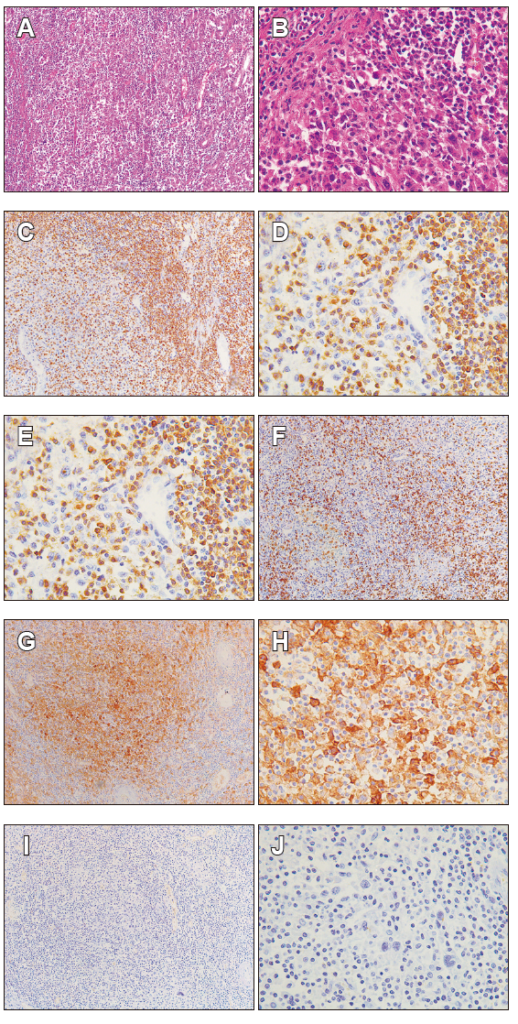
Figure 2. Representative images of advanced intestinal type adenocarcinoma findings. A. Hematoxylin & eosin staining shows the gastric antral mucosa (stomach), with a dense infiltrate TILs; B. It is detailed the presence of tumor cells accompanied by TILs. C and D. the presence of CD3+ lymphocyte by IHC. E and F. the presence of CD8+ lymphocyte by IHC. G and H. PDL-1 expression on cancer cells. I and J. PD-1 negative on T lymphocytes by IHC. All sections were appreciated at 10X and 40X magnification
EBV Expression by EBER-ISH in Advanced ITGA
The expression of EBV by EBER-ISH showed that 5 of the patients in the study (20.8%) presented infection by the virus in the cancer cells. The histopathological characteristics are shown in Table 2.
Table 2. Analysis of bivariate of EBER-ISH and pathological clinical characteristics of patients diagnosed with gastric adenocarcinoma of intestinal type 2014 -2016
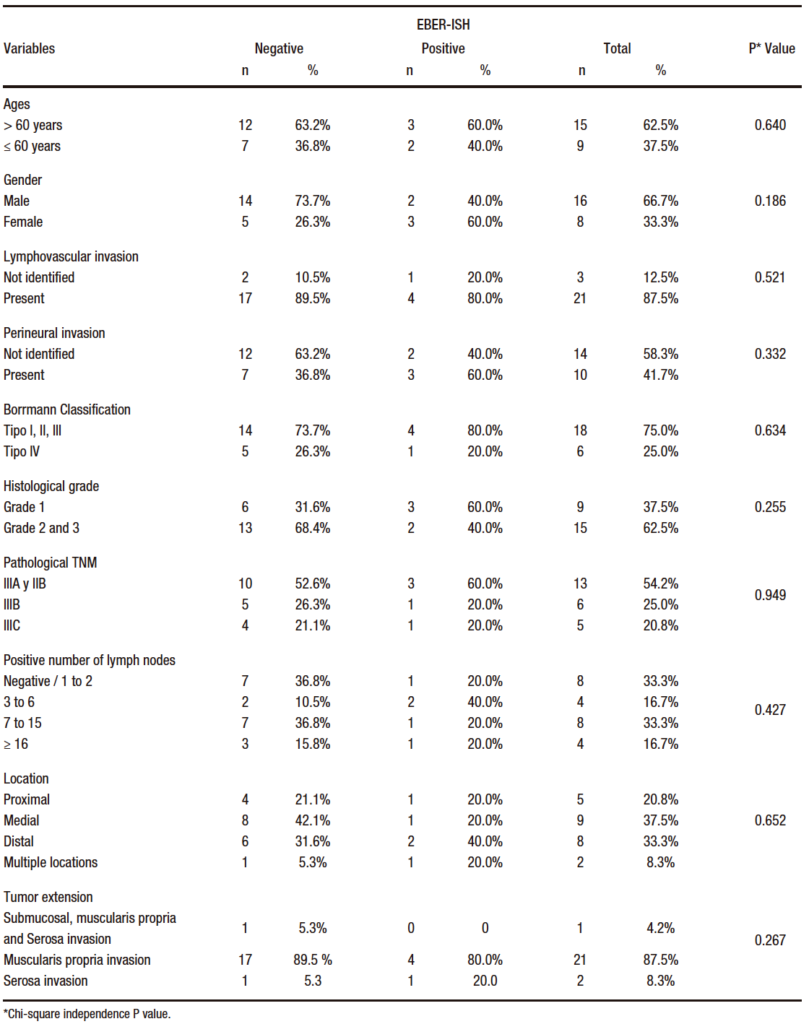
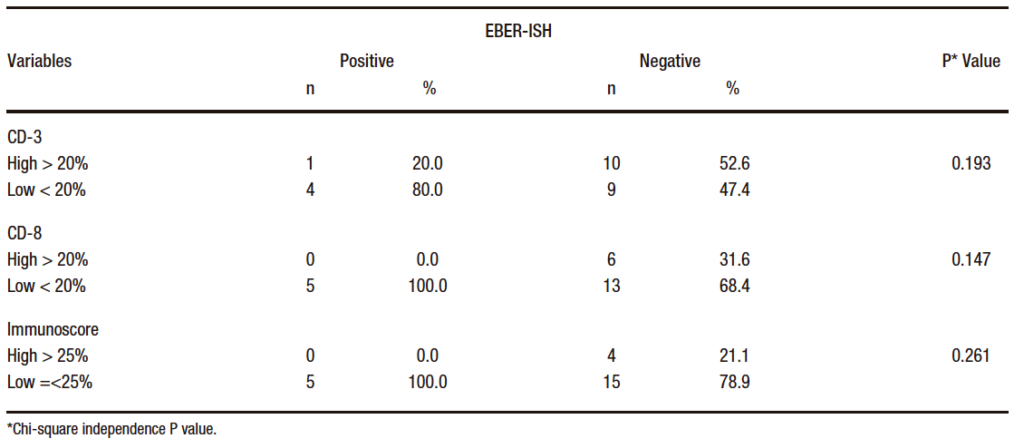
EBER-ISH positive patients (n= 5) reported low immunoscore (≤ 25%) and low CD8+ infiltration density (< 20%). 80% of the cases were low CD3+ (< 20%) and only 20% (n= 1) had high CD3+ (> 20%). Similarly, all positive cases for EBV presented low expression of PD-1 (< 20%) with a weak intensity of immunostaining and a < 1 CPS score. The percentage distribution of lymphoid infiltration is shown in Table 3.
Table 3. Distribution of EBER-ISH according to CD3+, CD8+ percentage, and immunoscore in samples of patients diagnosed with gastric adenocarcinoma of intestinal type 2014 -2016
Discussion
This study describes the CD3+ and CD8+ infiltration, the expression of PD-1 and PD-L1 proteins, and the EBV infection in 24 ITGA samples from a Colombian region with high incidence of GC and mortality. In the evaluation of clinical characteristics such as sex and age at diagnosis and histopathological findings of tumor lesions, it was noticed that those characteristics correlate with reported information found in the literature on advanced stage GC and GC associated with EBV.5, 18 The only exceptions are age and sex of EBER-ISH positive patients since it has been reported that GC associated with EBV is prevalent in male patients with a 2:1 or 3:1 ratio and occurs in people under 60 years old.18
In GC has been conducted the evaluation and quantification of TILs with the immunoscore value in which high immunoscore correlates with lower pT stage, post-surgical survival, and chemotherapeutic benefits.12, 19-21 In order to score the density of TILs microenvironment this study used the colon cancer immunoscore parameters, the only score validated to evaluate the count of T lymphocytes.11 20% of the patients presented a high immunoscore (≥ 25% score) and were classified in clinical stage III, as it was reported previously in advanced clinical conditions associated with high lymphoid infiltration.12, 21
In the immune checkpoint, PD-1and PD-L1 pathway plays a main role inside the tumoral microenvironment because the high expression of PD-1 on lymphocytes defines them as exhausted T cells and the expression of PD-L1 in tumor cells contributes to T cell inhibition when engages with PD-1.22 In recent years, that pathway has stood out as a target therapeutics in malignant neoplasms; for that reason, it is important to study the expression of both membrane proteins in a tumoral context to determine a therapeutic strategy.23 Although in the case of gastric adenocarcinoma, the cut-off point according to which PD-L1 is positive, could be considered useful for therapeutic purposes, this kind of analysis remains not clear yet.24 Specially because to these study, the ZR3 Zeta antibody clone, used for identification of PD-L1 expression, was only aimed at determining its expression and studying the type of immune response in the analyzed cases. In addition, we determined PD-L1 expression following the NCCN 2020 guidelines for the management of GC21 which indicates that the CPS is the most useful assessment method for this molecule.25 In this study, only 8.3% of the cases (n= 2) had a > 1 score, which also reported the highest percentages of CD3+ and CD8+ lymphoid infiltration. As a reporter by Xing et al. (2018), PD-L1 has high expression in tumor or immune cells. Its association with lymphocyte infiltration and further good outcome should be remarked.26 Previous studies have reported that PD-L1 expressions are consistent with advanced clinical stages.25, 27 However, in this study almost all samples were classified as a clinical stage between II and III, 91.7% had CPS < 1 score. The low PD- L1 expression would be explained by the absence of standardization of IHC, and by the antibodies clones variability available which brings conflicting results related to PDL1 expression.22, 26
As shown by Liang et al 2020, there was a variation in intensity staining and PD-L1 positive proportion of cells in advanced cervical cancer.28 These divergences could suggest the need to confirm the expression of PD-L1 by other molecular methods or using more than one clone of the antibody.
The EBV infection evaluated with colorimetric EBER-ISH technique showed that 20% of gastric samples were positive. This finding agrees with the worldwide estimation of EBV being responsible for 5.6%-19.5% of cases with GC.29 In the histopathological features, the EBV+ GC is associated with an abundant inflammatory infiltration by the presence of viral antigens. This is associated with a greater presentation of neoepitope, which contributes to an antitumor immune response.29 The high lymphocyte infiltration associated with EBV in GC has been reported by Cho et al 2018 who manifested that the cases of EBV+ GC are associated with a high density of intratumoral CD8+ cells, in comparison to its negative counterpart,30 and by Gong et al. (2019) who pointed out that the higher density of TILs was present in EBV+ GC.31 In this study, the EBV positive gastric samples present infiltration of CD3+ and CD8+ lymphocytes < 20%, that finding correlates with those reported by Cheng et al. (2022) where is shown that 52.4% and 35.7% of the EBV+ GC samples presented lymphocyte infiltration between < 10% and 10-50%, respectively.32 The lymphocyte infiltration in EBV+ GC is a typical feature of an antitumor immune response that can be translated into a good prognostic indicator.
Patients who were EBER-ISH negative presented high lymphocyte infiltration. Since this result is not associated with EBV infection. It is suggested that cancer in these patients could be related to MSI, another molecular subtype of GC that presents with abundant inflammatory infiltration, which occurs in response to the high rate of somatic mutations, which generate neoantigens that also cause an immune system response.33 The emerging knowledge about the prognostic and predictive role of MSI in GC has prompted in recent years to routinely test patients with GC to determine this molecular characteristic.34
The GC associated with EBV+ is characterized by the amplification of the CD247 gene that codes for the PD-L1 protein which leads to the robust expression of PD-L1.35 This PD-L1 overexpression has been evaluated and corroborated in several studies such as those published by Noh et al. 2018, Pereira et al. (2018) and Choy et al. (2018), who mention that PD-L1 overexpression was significantly associated with the EBV infection and the MSI status, compared to its negative counterpart.30, 36, 37
Although EBV positive patients did not have PD-L1 overexpression, as expected. This finding may be related to what was referred by Sundar et al. (2018) who stated that a substantial proportion of cases with GC associated with EBV does not express high levels of PD-L1 and other immune agents such as PD-1 because of a low transcriptomic expression of proteins.38 This situation could have occurred in our study.
In summary, the tumor microenvironment of patients with GC from a high incidence area of this disease shows atypical aspects, as in the positive EBER-ISH cases had < 20%, CD3+ and CD8+ lymphocytes infiltration, low immunoscore value, and PD-L1 low expression, while negative EBER-ISH cases had high immunoscore value. Thus, it is important, in the future, to consider studying other molecular aspects of cancer such as the MSI status to complement the molecular characterization of these patients. This study contributes to understanding the tumor microenvironment in GC and expects to promote new studies that characterize the different GC subtypes in Colombia, a country with one of the highest GC rates in Latin America.
Consent for Publication. Anonymized data were used for the elaboration of this article, which did not distort its scientific value.
Intellectual Property. The authors declare that the data, figures and tables that appear in this article are original and were made in their belonging institutions.
Funding. The author states that the Vice-rectory of research of Universidad del Cauca (identification code 5095) funded this study.
Conflict of interest. The author declares that he has no conflicts of interest in relation to this article.
Acknowledgments. We are grateful to the Hospital Universitario San José for the FFPE samples provided and the permission given to review clinical records, and the Vice-rectory of research of Universidad del Cauca, who contributed to financial of the resident of medical specialist in Anatomical pathology during the development of this work.
Copyright
© 2022 Acta Gastroenterológica Latinoamericana. This is an open-access article released under the terms of the Creative Commons Attribution (CC BY-NC-SA 4.0) license, which allows non-commercial use, distribution, and reproduction, provided the original author and source are acknowledged.
Cite this article as: Bravo-Luna L, Dueñas-Cuellar R A, Niño-Castaño V E et al. Descriptive Study of Tumor Immune Microenvironment and Epstein-Barr Virus Infection in Gastric Adenocarcinoma. Acta Gastroenterol Latinoam. 2022;52(4):484-493. https://doi.org/10.52787/agl.v52i4.200
References
- Liu X, Meltzer SJ. Gastric cancer in the era of precision medicine. Cellular and molecular gastroenterology and hepatology. 2017;3(3):348-58.
- Sierra MS, Cueva P, Bravo LE, Forman D. Stomach cancer burden in Central and South America. Cancer epidemiology. 2016;44:S62-S73.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71(3):209-49.
- Ospina ML, Huertas JA, Montaño JI, Rivillas JC. Observatorio nacional de cáncer Colombia. Revista Facultad Nacional de Salud Pública. 2015;33(2):262-76.
- Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182.
- Asakawa Y, Okabe A, Fukuyo M, Li W, Ikeda E, Mano Y, et al. Epstein-Barr virus-positive gastric cancer involves enhancer activation through activating transcription factor 3. Cancer science. 2020;111(5):1818.
- Sohn BH, Hwang J-E, Jang H-J, Lee H-S, Oh SC, Shim J-J, et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clinical Cancer Research. 2017;23(15):4441-9.
- Kim TS, Da Silva E, Coit DG, Tang LH. Intratumoral immune response to gastric cancer varies by molecular and histologic subtype. The American journal of surgical pathology. 2019;43(6):851.
- Vareki SM, Garrigós C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Critical reviews in oncology/hematology. 2017;116:116-24.
- Dabbs DJ. Diagnostic Immunohistochemistry E-Book: Theranostic and Genomic Applications: Elsevier Health Sciences; 2017.
- Pagès F, Mlecnik B, Marliot F, Bindea G, Ou F-S, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. The Lancet. 2018;391(10135):2128-39.
- Yun S, Koh J, Nam SK, Kwak Y, Ahn S-H, Do Park J, et al. Immunoscore is a strong predictor of survival in the prognosis of stage II/III gastric cancer patients following 5-FU-based adjuvant chemotherapy. Cancer Immunology, Immunotherapy. 2021;70(2):431-41.
- Mlecnik B, Bifulco C, Bindea G, Marliot F, Lugli A, Lee JJ, et al. Multicenter International Society for Immunotherapy of Cancer study of the consensus Immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol. 2020:3638-51.
- Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nature Reviews Cancer. 2020;20(11):662-80.
- Yuan Y, Jiang Y-C, Sun C-K, Chen Q-M. Role of the tumor microenvironment in tumor progression and the clinical applications. Oncology reports. 2016;35(5):2499-515.
- Network NCC. National Comprehensive Cancer Network (NCCN) Guidelines. 2015.
- Wen T, Wang Z, Li Y, Li Z, Che X, Fan Y, et al. A four-factor immunoscore system that predicts clinical outcome for stage II/III gastric cancer. Cancer immunology research. 2017;5(7):524-34.
- Naseem M, Barzi A, Brezden-Masley C, Puccini A, Berger MD, Tokunaga R, et al. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer treatment reviews. 2018;66:15-22.
- Zeng D, Zhou R, Yu Y, Luo Y, Zhang J, Sun H, et al. Gene expression profiles for a prognostic immunoscore in gastric cancer. Journal of British Surgery. 2018;105(10):1338-48.
- Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, et al. ImmunoScore signature: a prognostic and predictive tool in gastric cancer. Annals of surgery. 2018;267(3):504-13.
- Jiang Y, Wang H, Wu J, Chen C, Yuan Q, Huang W, et al. Noninvasive imaging evaluation of tumor immune microenvironment to predict outcomes in gastric cancer. Annals of Oncology. 2020;31(6):760-8.
- Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets and therapy. 2016;9:5023.
- Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434-52.
- Xie T, Zhang Z, Zhang X, Qi C, Shen L, Peng Z. Appropriate PD-L1 cutoff value for gastric cancer immunotherapy: a systematic review and meta-analysis. Frontiers in oncology. 2021;11:646355.
- Yamashita K, Iwatsuki M, Harada K, Eto K, Hiyoshi Y, Ishimoto T, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer. 2020;23(1):95-104.
- Xing X, Guo J, Ding G, Li B, Dong B, Feng Q, et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology. 2018;7(3):e1356144.
- Koh J, Ock C-Y, Kim JW, Nam SK, Kwak Y, Yun S, et al. Clinicopathologic implications of immune classification by PD-L1 expression and CD8-positive tumor-infiltrating lymphocytes in stage II and III gastric cancer patients. Oncotarget. 2017;8(16):26356.
- Liang Y, Yu M, Zhou C, Zhu X. Variation of PD-L1 expression in locally advanced cervical cancer following neoadjuvant chemotherapy. Diagnostic Pathology. 2020;15:1-8.
- Wang H, Chen X-L, Liu K, Bai D, Zhang W-H, Chen X-Z, et al. Associations between gastric cancer risk and virus infection other than epstein-barr virus: a systematic review and meta-analysis based on epidemiological studies. Clinical and translational gastroenterology. 2020;11(7).
- Cho CJ, Kang HJ, Ryu Y-M, Park YS, Jeong HJ, Park Y-M, et al. Poor prognosis in Epstein–Barr virus-negative gastric cancer with lymphoid stroma is associated with immune phenotype. Gastric Cancer. 2018;21(6):925-35.
- Gong L-p, Chen J-n, Xiao L, He Q, Feng Z-y, Zhang Z-g, et al. The implication of tumor-infiltrating lymphocytes in Epstein-Barr virus–associated gastric carcinoma. Human pathology. 2019;85:82-91.
- Cheng N, Li P, Cheng H, Zhao X, Dong M, Zhang Y, et al. Prognostic Value of Tumor-Infiltrating Lymphocytes and Tertiary Lymphoid Structures in Epstein-Barr Virus-Associated and-Negative Gastric Carcinoma. Frontiers in immunology. 2021;12:2560.
- Rodriquenz MG, Roviello G, D’Angelo A, Lavacchi D, Roviello F, Polom K. MSI and EBV positive gastric cancer’s subgroups and their link with novel immunotherapy. Journal of clinical medicine. 2020;9(5):1427.
- Ratti M, Lampis A, Hahne JC, Passalacqua R, Valeri N. Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches. Cellular and Molecular Life Sciences. 2018;75(22):4151-62.
- Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7(22):32925.
- Noh B-J, Kim JH, Eom D-W. Prognostic significance of categorizing gastric carcinoma by PD-L1 expression and tumor infiltrating lymphocytes. Annals of Clinical & Laboratory Science. 2018;48(6):695-706.
- Pereira MA, Ramos MF, Faraj SF, Dias AR, Yagi OK, Zilberstein B, et al. Clinicopathological and prognostic features of Epstein-Barr virus infection, microsatellite instability, and PD-L1 expression in gastric cancer. Journal of surgical oncology. 2018;117(5):829-39.
- Sundar R, Qamra A, Tan ALK, Zhang S, Ng CCY, Teh BT, et al. Transcriptional analysis of immune genes in Epstein–Barr virus-associated gastric cancer and association with clinical outcomes. Gastric Cancer. 2018;21(6):1064-70.
Correspondence: Harold Bolaños
Email: haroldbolanos@unicauca.edu.co
Acta Gastroenterol Latinoam 2022;52(4):484-493
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE