Joana C Branco, Ana M Oliveira, David Horta, Jorge Reis
Gastroenterology Department/Servicio de Gastroenterologia – Hospital Professor Doutor Fernando Fonseca. Amadora, Portugal.
Acta Gastroenterol Latinoam 2019;49(2):150-152
Recibido: 17/01/2018 / Aprobado: 05/12/2018 / Publicado en www.actagastro.org el 17/06/2019
Summary
Raoultella planticola are Gram-negative bacteria that cause very rarely infections and affect mainly immunocompromised patients. We present the case of a 76-year-old man, with past medical history of alcoholic liver cirrhosis Child-Pugh B and insulin-dependent type 2 diabetes mellitus that was diagnosed with spontaneous bacterial peritonitis caused by R. planticola, which was successfully treated with ceftriaxone. To our knowledge this is the second documented report of R. planticola infection in a cirrhotic patient and spontaneous bacterial peritonitis.
Key words. Peritonitis, alcoholic liver cirrhosis, gram-negative bacteria.
Peritonitis bacteriana espontánea causada por Raoultella planticola – Caso raro con revisión de la literatura
Resumen
Raoultella planticola es una bacteria Gram-negativa que raramente causa infecciones y afecta especialmente a pacientes inmunocomprometidos. Se presenta el caso de un paciente de sexo masculino, de 76 años, con antecedentes personales de cirrosis hepática alcohólica Child-Pugh B y diabetes mellitus tipo 2 insulinotratada, con diagnóstico de peritonitis bacteriana espontánea causada por R. planticola, tratada con éxito con ceftriaxone. Después de la revisión bibliográfica hemos constatado que este es el segundo caso de infección documentada en un paciente con cirrosis hepática por R. planticola y peritonitis bacteriana espontánea.
Palabras claves. Peritonitis, cirrosis hepática alcohólica, bacterias gram-negativas.
Abreviaturas
R. planticola: Raoultella planticola
SBP: spontaneous bacterial peritonitis
Raoultella planticola (R. planticola) are Gram-negative bacteria that cause essentially opportunistic infections in immunocompromised patients as they are not a highly virulent pathogen.1 They are emerging pathogens and the number of case reports relating its infectious potential has been increasing over the past years. There is yet only one supposed report of a spontaneous bacterial peritonitis (SBP) to this microorganism. In regard to this, our aim is to describe the second case report of an SBP to R. planticola and to review the literature about R. planticola infections.
Case report
A 76-year-old man was hospitalized in the gastroenterology ward for ascites and dyspnea. His past medical history was relevant for alcoholic liver cirrhosis (now abstinent) Child-Pugh B (8 points) with portal hypertension and a previous episode of decompensation by esophageal varices rupture, with a regular follow-up in the gastroenterology consultation, with no previous history of SBP. He also had insulin-dependent type 2 diabetes mellitus. He was medicated with furosemide, spironolactone, lactitol and insulin.
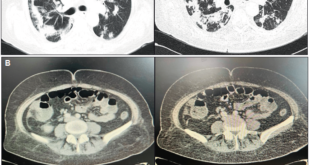
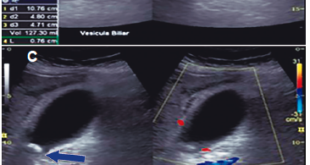
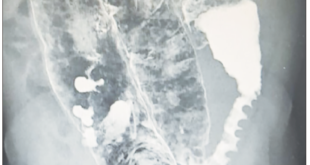
The patient has presented to the Emergency Department after a fall from his own height which resulted in a trochanteric fracture of the left femur. As he showed concomitantly ascites and a right pleural effusion, with consequent dyspnea, he was transferred to the gastroenterology ward for preoperative stabilization. At the physical exam he had hepatic encephalopathy grade 1, diminished vesicular murmur on the bottom half of the right thorax and tension ascites. Laboratory blood tests showed a normocytic normochromic anemia with a hemoglobin of 10.7 g/dL, thrombocytopenia of 39000/uL, a prolonged INR of 1.6, normal liver tests except for elevated total bilirubin, of 2.4 mg/dL (at the expense of the unconjugated fraction), hypoalbuminemia of 2.8 g/dL, creatinine of 1.7 mg/dL, urea of 45 mg/dL and C-reactive protein of 2.6 mg/dL. At this point, he had a Child-Pugh score of 10 (class C) and a MELD of 20. Chest X-ray showed a right pleural effusion on the bottom half of the right thorax, with no consolidations or images suggestive of pneumonia. We performed both paracentesis and thoracocentesis. In the ascitic fluid the serum-ascites albumin gradient was 0.9, suggestive of portal hypertension and 3394 neutrophils/uL were observed, which was compatible with a SBP. On the cultural microbiologic exam (in Bactec) R. planticola was isolated, sensitive to ceftriaxone, with no other microrganisms. In the pleural fluid (PF) showed a PF/serum protein of 0.2, PF/serum LDH of 0.3 and a PF LDH 2/3 inferior to the serum LDH, characteristics suggestive of a hepatic hydrothorax, and the microbiologic exam was negative. Urine and blood cultures were also negative. The patient completed seven days of ceftriaxone and the standard protocol of albumin for SBP with a good clinical and laboratorial response, namely a fall of 30% in the neutrophils of the ascitic fluid 48 hours after the initiation of the antibiotic. The patient achieved reasonable ventilatory conditions and was transferred to the orthopedics ward and submitted to a reduction of the fracture. Nevertheless, he developed nosocomial pneumonia to Escherichia coli which lead to acute-on-chronic liver failure stage 3 and, subsequently, his death.
Discussion
The bacteria of the genus Raoultella are Enterobacteriacae that have been isolated in various environments such as plants, soil, water, food, animals and humans,1 where they can colonize the upper respiratory or the digestive tracts2 – rarely both – and they can also survive in the hospital environments.3 Four species belong to the genus: R. planticola, Raoultella ornithinolytica, Raoultella terrigena and Raoultella electrica.1 Raoultella spp. have been recognized as increasingly important pathogens in recent years. The most important – for its clinical significance – are R. planticola and R. ornithinolytica strains.1 Raoultella is not a highly virulent pathogen and the pathogenesis of infection is related to the presence of lipopolysaccharide, polysaccharide capsule, fimbriae, siderophores, toxin, hydrolytic enzymes, bacteriocins and its ability to form biofilm.1
After the identification of R. planticola in 1981, the first report of a human infection was published in 1984 in a patient with sepsis4 and until December 2017 there have been 45 published case reports,1, 5-19 and there is also a recent case series which comprises 42 cases of infection by this microorganism.10 The infection sites are listed by frequency: bloodstream infections (32 cases), urinary tract infection (16 cases), pneumonia (11 cases), cholangitis (5 cases), pancreatitis (3 cases), cholecystitis (3 cases), conjunctivitis (2 cases), surgical site infection (3 cases), secondary bacterial peritonitis (2 cases), joint infection (2 cases), prostatitis (1 case), cellulitis (1 case), necrotizing fasciitis (1 case), enterocolitis (1 case), oral mucositis (1 case), infection of a cardiac implantable electronic device (1 case), liver abscess (1 case) and dialysis-related peritonitis (1 case).
Another important aspect to emphasize is the immunocompromised state of almost all patients, with considerable underlying comorbidities. The main pointed risk factors for infection by these genera are: 1, 14 enteral feeding tubes; premature, newborns and infants; prolonged hospitalization in ICU; long-term antibiotic therapy; chemotherapy and cancer; steroid use; diabetes mellitus and chronic renal insufficiency; endoscopic procedure and catheters. Until now, there has been only one report of this infection in a cirrhotic patient, presumably due to SBP,16 although infections in cirrhosis are prevalent, in particular SBP, which affects 10 to 33% of cirrhotic hospitalized patients and portends high short-term mortality,20 and is mainly related to renal dysfunction and elevated MELD score (both present in this patient).
R. planticola is usually antibiotic sensitive and these infections have typically been treated with third – to fourth-generation cephalosporin’s (as in this case report) or fluoroquinolones (alone or with aminoglycosides). Nevertheless, they can acquire genes of antibiotic resistance, which may affect an increase in isolation of multidrug-resistant strains.1
With this case we pretend to advertise for two important aspects of the infection by R. planticola: the potential of infecting cirrhotic patients – which constitutes a state of immunosuppression – and of causing SBP, a severe infection in these patients.
Authors’ contributions – according to ICMJE criteria for authorship:
Concept and design: JCB, JR.
Analysis and interpretation of the data: JCB, AO.
Article writing: JCB, AO, DH.
Content review: DH, JR.
Final approval: JR.
Conflict of interest disclosure. All authors report having NO conflicts of interest.
Funding sources. This work has NOT received any funding sources.
Contribuiciones de los autores – según critérios de ICMJE para autoría:
Concepto y diseno: JCB, JR.
Análisis y interpretación de los datos: JCB, AO.
Escritura del artículo: JCB, AO, DH.
Revisión del contenido: DH, JR.
Aprobación finale: JR.
Conflicto de intereses. Los autores declaran no tener ningún conflicto de interés.
Sostén financiero. No se solicitó ningún apoyo financiero para la elaboración del caso clínico.
References
- Sekowska A. Raoutella spp. – clinical significance, infections and susceptibility to antibiotics. Folia Microbiol 2017; 62: 221-227.
- Podschun R, Acktun H, Okpara J, Linderkamp O, Ullmann U, Borneff Lipp M. Isolation of Klebsiella planticola from newborns in a neonatal ward. J Clin Microbiol 1998; 36: 2331-2332.
- Freney J, Gavini F, Alexandre H, Madier S, Izard D, Leclerc H, Fleurette J. Nosocomial infection and colonization by Klebsiella trevisanii. J Clin Microbiol 1986; 23: 948-950.
- Freney J, Fleurette J, Gruer LD, Desmonceaux M, Gavini F, Leclerc H. Klebsiella trevisanii colonisation and septicaemia. Lancet 1984; 21: 909.
- Sitaula S, Shahrrava A, Al Zoubi M, Malow J. The first case report of Raoultella planticola liver abscess. IDCases 2016; 5: 69-71.
- de Campos FP, Guimarães TB, Lovisolo SM. Fatal pancreatic pseudo cyst co-infected by Raoultella planticola: an emerging pathogen. Autops Case Rep 2016; 6: 27-33.
- Sia CS, Wilson S, Ananda-Rajah M, Mills J, Aung AK. Refractory Raoultella planticola peritonitis in a HIV positive patient. Nephrology (Carlton) 2016; 21: 979-980.
- Pan Z, Liu R, Zhang P, Zhou H, Fu Y, Zhou J. Combination of tigecycline and levofloxacin for successful treatment of nosocomial pneumonia caused by a New Delhi metallo-beta-lactamase- 1-producing Raoultella planticola. Microb Drug Resist 2017; 23: 127-131.
- Merino Rodríguez E, Rebolledo Olmedo S, Miquel Plaza J. Bacteremia with Raoultella planticola in the setting of acute pancreatitis complicated with acute cholangitis. Rev Esp Enferm Dig 2017; 109: 479.
- Adjodah C, D’Ivernois C, Leyssene D, Berneau JB, Hemery Y. A cardiac implantable device infection by Raoultella planticola in an immunocompromized patient. JMM Case Rep 2017; 4: e005080.
- Bardellini E, Amadori F, Schumacher RF, Foresti I, Majorana A. A new emerging oral infection: Raoultella planticola in a boy with haematological malignancy. Eur Arch Paediatric Dent 2017; 18: 215-218.
- Atıcı S, Alp Ünkar Z, Öcal Demir S, Akkoç G, Yakut N, Yılmaz Ş, Erdem K, Çınar Memişoğlu A, Ülger N, Soysal A, Özek E, Bakır M. A rare and emerging pathogen: Raoultella planticola identification based on 16S rRNA in an infant. J Infect Public Health 2018; 11: 130-132.
- Ulukent SC, Sarici İS, Alper Sahbaz N, Ozgun YM, Akca O, Sanlı K. Is it necessary to specifically define the cause of surgically treated biliary tract infections? a rare case of Raoultella planticola Cholecystitis and Literature Review. Case Rep Infect Dis 2017; 2017: 4181582.
- Demiray T, Koroglu M, Ozbek A, Altindis M. A rare cause of infection, Raoultella planticola: emerging threat and new reservoir for carbapenem resistance. Infection 2016; 44: 713-717.
- Subedi R, Dean R, Li W, Dhamoon A. A novel case of Raoultella planticola osteomyelitis and epidural abscess. BMJ Case Rep 2017; 2017.
- Povlow MR, Carrizosa J, Jones A. Raoultella planticola: bacteremia and sepsis in a patient with cirrhosis. Cureus 2017; 9: e1508.
- Yoshida N, Tsuchida Y. Palm atheroma infection caused by Raoultella plancticola. BMJ Case Rep 2017; 2017.
- Kalaria SS, Elliott K, Combs N, Phillips LG. Raoultella planticola: a rare cause of wound infection. 2017; 29: E103- E105.
- Yamamoto S, Nagatani K, Sato T, Ajima T, Minota S. Raoultella planticola bacteremia in a patient with early gastric cancer. Intern Med 2018; 57: 1469-1473.
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010; 53: 397-417.
Correspondencia: Andrés José Gómez Aldana
Carrera 7 No 117 – 15. Bogotá, Colombia
Tel.: +57 3112139546
Correo electrónico: andresgomezmd@hotmail.com
Acta Gastroenterol Latinoam 2019;49(2): 150-152
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE