Carlos Eduardo Oliveira dos Santos,1 Júlio Carlos Pereira-Lima,2 Daniele Malaman,1 Tiago dos Santos Carvalho,1 Michele Lemos Bonotto2
1 Department of Endoscopy and Gastroenterology, Santa Casa de Caridade Hospital. Bagé, RS, Brazil.
2 Department of Gastroenterology and Endoscopy, Santa Casa Hospital. Porto Alegre, RS, Brazil.
Acta Gastroenterol Latinoam 2016;46: 184-191
Recibido: 31/08/2015 / Aprobado: 09/05/2016 / Publicado en www.actagastro.org el 03/10/2016
Summary
This study aims to assess the prevalence and clinicopathologic features of colorectal laterally spreading tumors (LSTs). Methods. Among 2067 superficial neoplastic lesions diagnosed in 1135 consecutive patients, we evaluated 91 patients with 96 LSTs classified as granular (homogeneous and nodular mixed) and non-granular (flat elevated and pseudodepressed) types. Lesions were evaluated according to their clinicopathologic features. LSTs ≤ 20 mm were resected en bloc and LSTs > 20 mm by piecemeal resection. Recurrence was analyzed. Results. Overall prevalence of LSTs was 2.5% among all colonoscopies, accounting for 4.6% of superficial neoplastic lesions. Of 96 LSTs, 68 (70.8%) were granular (42 homogeneous and 26 nodular mixed). Among non-granular LSTs, 21 were flat elevated and 7 were pseudodepressed. Granular LSTs of the nodular mixed subtype were significantly larger than those of the homogeneous subtype (p < 0.01), with no difference in size between non-granular subtypes. Twenty-three (24%) LSTs had advanced histology. Logistic regression analysis showed that lesion size > 20 mm (OR: 12.2; IC 95%: 3.8-39.2) and pseudo-depressed (OR: 8.0; IC 95%: 1.2-55.3) and especially nodular mixed (OR: 23.3; IC 95%: 4.6-117.4) subtypes were risk factors for advanced histology. All patients underwent endoscopic mucosal resection, which was complemented with surgical intervention in one case. Recurrence occurred in 6.4% (5/78) of cases and was associated with lesions > 20 mm removed by piecemeal resection (p = 0.003) and with advanced histology (p = 0.009). Conclusions. LST accounted for 4.6% of superficial neoplastic lesions and 10% of non-polypoid neoplasms. Those lesions > 20 mm pseudo-depressed and nodular mixed subtypes were considered risk factors for advanced histology. Endoscopic mucosal resection, even using the piecemeal technique, is still a fair option for the treatment of LSTs.
Key words. Colorectal laterally spreading tumors, piecemeal technique, LST.
Prevalencia y características clínico-patológicas de tumores colorrectales de crecimiento lateral en Brasil Latina
Resumen
Este estudio tiene como objetivo evaluar la prevalencia y las características clínico-patológicas de los tumores colorrectales de crecimiento lateral (LST). Material y métodos. De las 2067 lesiones neoplásicas superficiales diagnosticadas en 1135 pacientes consecutivos, nosotros evaluamos 91 pacientes con 96 LST clasificados como granulares (homogénea y nodular mixta) y no granulares (elevados y pseudo depresiones planas). Las lesiones fueron evaluadas de acuerdo a sus características clínico-patológicas. LST ≤ 20 mm fueron resecadas en bloque y LST > 20 mm mediante resección fragmentaria. La recurrencia fue analizada. Resultados. La prevalencia global de LST fue 2,5% entre todas las colonoscopias, que representan el 4,6% de las lesiones neoplásicas superficiales. De los 96 LSTs, 68 (70,8%) eran de tipo granular (42 homogénea y 26 nodular mixto). Entre LST de tipo no granulares, 21 se mantuvieron elevados y 7 fueron pseudo-depresión plana. LSTs granular del subtipo nodular mixto fueron significativamente mayores que los del subtipo homogéneo (p < 0,01), sin diferencia en el tamaño entre los subtipos no granulares. Veinte y tres (24%) LSTs tenían histología avanzada. El análisis de regresión logística mostró que la lesión con tamaño > 20 mm (OR: 12,2; IC 95%: 3,8-39,2), con pseudo-depresión (OR: 8,0; IC 95%: 1,2-55,3) y los subtipos especialmente nodular mixtos (OR: 23,3; IC 95%: 4,6-117,4) fueron factores de riesgo para histología avanzada. Todos los pacientes fueron sometidos a resección endoscópica de la mucosa, que se completó con una intervención quirúrgica en un caso. La recurrencia se produjo en el 6,4% (5/78) de los casos y se asoció con lesiones > 20 mm removidas por resección fragmentaria (p = 0,003) y con histología avanzada (p = 0,009). Conclusiones. LST representó el 4,6% de las lesiones neoplásicas superficiales y el 10% de las neoplasias no polipoides, aquellas lesiones > 20 mm subtipos pseudo-depresiones y nodulares mixtos fueron considerados factores de riesgo para la histología avanzada. La resección endoscópica de la mucosa utilizando la técnica piecemeal es una opción adecuada para el tratamiento de LST.
Palabras claves. Tumores colorrectales, crecimiento lateral, LST, piecemeal technique.
Colorectal cancer is currently one of the fastest growing malignant diseases in both Western and Eastern countries. Endoscopic resection of premalignant lesions significantly reduces the incidence of colorectal cancer.1
Three pathways have been recognized as having a role in the evolution of colorectal cancer: the adenoma-carcinoma sequence,2 the theory of de novo cancer,3, 4 and the serrated pathway,5, 6 the latter with an important participation of sessile serrated adenoma, which tends to be larger and have a flat morphology, preferably located in the right colon and coated with mucin.7
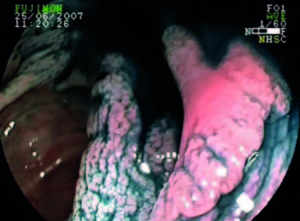
Laterally spreading tumors (LSTs) are non-polypoid lesions, generally defined as flat lesions ≥ 10 mm in diameter, which are characterized by a lateral and circumferential growth in the colonic wall, with deep invasion of the submucosa occurring only at later stages. LSTs are classified as granular (LST-G) and non-granular (LST-NG) types, according to the presence or absence of a granular surface pattern. Kudo et al8 proposed a sub-classification of LSTs, in which LST-Gs are classified as homogeneous and nodular mixed subtypes, and LST-NGs are classified as flat elevated and pseudodepressed subtypes. The nodular mixed-subtype LST-G and the pseudodepressed-subtype LST-NG have a higher potential for malignancy.9 The rate of invasive carcinoma in LSTs is low, and most cases are considered adenomatous lesions, where the homogeneous subtype tends to be a tubular adenoma, and the nodular mixed subtype tends to have villous features.10
In most cases, the treatment of choice is endoscopic resection, while surgical resection is indicated for lesions with massive submucosal invasion. Studies have shown that pit and capillary pattern analysis using image magnification is useful in differentiating between neoplastic and non-neoplastic lesions and in diagnosing early invasive colorectal cancer, factors that can help determine the therapeutic approach.11-16
The present study aimed to assess the prevalence and clinicopathologic features of LSTs.
Material and methods
This was a prospective, cross-sectional, observational study of 2841 superficial colorectal lesions diagnosed in 1675 patients (31.3%) among 3623 consecutive colonoscopies, in which magnification and real or virtual chromoscopy was always available, performed from January 2007 to December 2011 in the Department of Endoscopy at Hospital Santa Casa de Caridade de Bagé, Southern Brazil. Of the total sample, 2067 (72.8%) were superficial neoplastic lesions, detected in 1135 patients, and 961 (53.5%) were non-polypoid lesions, detected in 542 patients. Ninety-six LSTs were detected in 91 patients, and the following clinicopathologic variables were analyzed: patient sex and age, endoscopic findings (morphological type and subtypes, size, site, and pit and capillary patterns), histology (adenoma with grade of dysplasia, or early cancer), and recurrence. Lesions were termed superficial when their endoscopic appearance suggested that depth was limited to the mucosa or submucosa. Exclusion criteria were inadequate bowel preparation, incomplete colonoscopy, coagulopathy, presence of inflammatory bowel disease, polyposis syndrome, advanced cancer, and previous colorectal resection.
All colonoscopies were performed by a single endoscopist (CEOS) who had great experience in colonoscopy and routinely uses magnification and chromoendoscopy.
The study was approved by the Research Ethics Committee of Hospital Santa Casa de Caridade de Bagé and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients included in the study, and patient anonymity was preserved.
All patients were examined with a Fujinon 490ZW5 or 590ZW5 high-resolution magnifying video colonoscope (Fujifilm Corp, Saitama, Japan) equipped with an EPX 4400 processor. We routinely used magnification and real-time or digital chromoendoscopy in order to delineate lesions, determine their morphology, analyze their pit and capillary patterns, and thus make a differential diagnosis between neoplastic and non-neoplastic lesions in order to choose the most appropriate approach for patients. The Kudo classification17, 18 was used as a reference for pit pattern analysis and the Teixeira classification19 for capillary pattern analysis. In both classifications, types III, IV, and V suggested neoplastic lesions. Chromoendoscopy with indigo carmine was used in pseudodepressed-subtype lesions.
Bowel preparation consisted of one-day clear liquid diet and 10% mannitol solution administered on the day of examination, and was considered appropriate in all patients included in the study. Conscious sedation was achieved with administration of midazolam and meperidine or fentanyl before examination.
Lesion characteristics
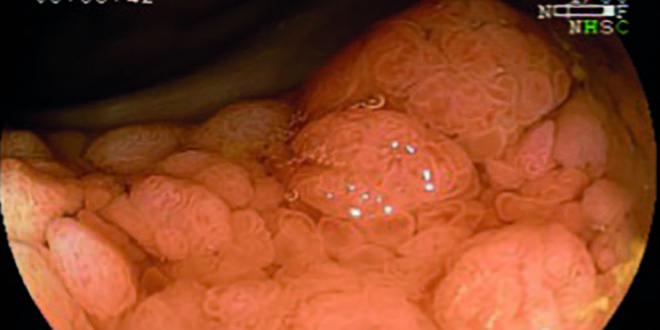
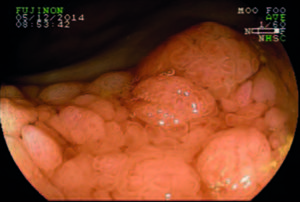
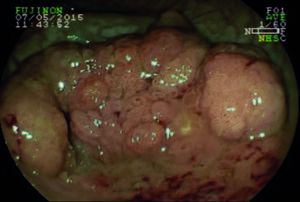
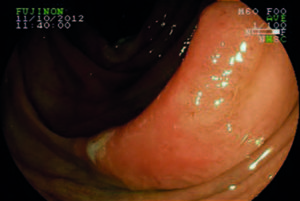
Lesion morphology was determined using the Paris classification.20 LSTs were defined as flat lesions ≥ 10 mm in diameter and characterized by more horizontal than vertical growth, with absent or shallow invasion of the submucosa. LSTs were classified as granular (LST-G) and non-granular (LST-NG) types, and each type was divided into two subtypes.8 LST-G was divided into homogeneous (LST-G-H) and nodular mixed (LST-G-N) subtypes, and LST-NG into flat elevated (LST-NG-FE) and pseudodepressed (LST-NG-PD) subtypes (Figures 1-4). In LST-G, the homogeneous subtype appears endoscopically as a type 0-IIa, whereas the nodular mixed subtype appears as a mixture of types 0-IIa and 0-Is. In LST-NG, the flat elevated subtype has a smooth surface and the pseudodepressed subtype appears as a mixture of types 0-IIc and 0-IIa, with a smooth surface as well.
LSTs are considered non-polypoid lesions because LST-G-H, LST-NG-FE, and LST-NG-PD subtypes do not exceed 2.5 mm in height, as estimated by comparison with the height of the closed cups of a biopsy forceps placed at the margin of the lesion. The LST-G-N subtype, however, exceeds 2.5 mm in height. In this case, the LST is considered a non-polypoid lesion because its maximum height does not exceed one-third of its horizontal width (height-to-width ratio < 1:3). Lesion size was estimated by comparing it with a fully opened biopsy forceps and confirmed after resection.
Lesions were divided into sessile serrated adenomas, adenomas with low-grade dysplasia, and neoplasms with advanced histology. Advanced histology was defined as lesions with high-grade dysplasia or early cancer.
All specimens were analyzed by a single pathologist who was blinded to the endoscopic findings. Lesions ≤ 20 mm were resected en bloc, whereas lesions > 20 mm were removed by piecemeal endoscopic mucosal resection (EMR) in a single session.
For histological analysis, specimens were mounted on Styrofoam plates and then fixed in 10% formalin. Resection was considered complete if vertical and lateral margins were free of tumor, and incomplete when there was evidence of malignancy in the lateral or deep margins. The right colon included the transverse colon, ascending colon, and cecum, whereas the left colon included the rectum, sigmoid colon, and descending colon.
Recurrence (or residual neoplasm) was defined as the presence of neoplastic tissue in the area of previous resection, as diagnosed by follow-up colonoscopy. Patients underwent follow-up colonoscopy at 6, 12, and 18 months. Recurrent/residual mucosal lesions were treated with a second EMR and identified by the scar, whereas lesions with massive submucosal invasion were referred for surgery.
Statistical analysis
Data were entered into an Excel spreadsheet and subsequently exported to Stata, version 11.2, for analysis. Descriptive analysis consisted of calculating absolute and relative frequencies (for categorical variables) and means and standard deviations (for numerical variables). Bivariate analysis was performed using Fisher’s exact test for categorical variables and analysis of variance (ANOVA) for numerical variables. A simple logistic regression model was then generated to analyze characteristics associated with the diagnosis of advanced histology, and odds ratios (OR) are shown with their respective 95% confidence intervals (95% CI). The level of significance was set at 5% for two-tailed tests.
Results
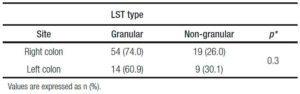
The overall prevalence of LSTs was 2.5% among all colonoscopies, 8% of patients with premalignant colorectal lesions, and 16.8% of patients with non-polypoid lesions. LSTs accounted for 4.6% of superficial neoplastic lesions and 10% of non-polypoid neoplasms. Of 91 patients with 96 LSTs, 63 (69.2%) were women. Eighty-three (91.2%) patients were ≥ 50 years of age, and mean patient age was 67 ± 13 years. Eighteen (18.8%) LSTs were > 20 mm, and mean lesion size was 16.9 ± 8.1 mm. The characteristics of patients and LSTs are shown in Table 1.
Table 1. Clinicopathologic features of laterally spreading tumors (LSTs).

LST-G accounted for 70,8% of lesions (n = 68, 42 LST-G-H and 28 LST-G-N), which were of similar size to LST-NG (n = 28; 21 LST-NG-FE and 7 LST-NG-PD): 17.4 ± 8.9 mm vs 15.6 ± 5.4 mm (p = 0.3). However, LST-G-Ns were larger than LST-G-Hs (22.3 ± 8.1 mm vs 14.4 ± 8.1 mm; p < 0.01). There was no difference between LST-NG-FE and LST-NG-PD (15.7 ± 5.6 mm vs 15.4 ± 5.3 mm; p = 0.9).
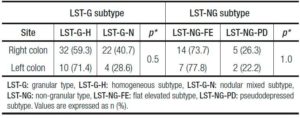
LSTs were more commonly located in the ascending colon (39.6%), followed by the cecum (29.2%) and rectum (12.5%), thus predominating in the right colon (73.8%). When analyzing the association between LST site (right vs left colon) and morphology, no difference was observed between LST-G and LST-NG types (p = 0.3) or between LST-G-H vs LST-G-H-N subtypes (p = 0.5) and LST-NG-FE vs LST-NG-PD subtypes (p = 1.0) (Tables 2 and 3).
Regarding the capillary pattern of LST-G, 77.8% (35/45) of type III capillary pattern was found in the LST-G-H subtype, and 69.6% (16/23) of type IV capillary pattern was diagnosed in the LST-G-N subtype (p < 0.001). In LST-NG-FE, we observed 70.6% (12/17) of type III capillary pattern, 100% (3/3) of type IV capillary pattern, and 100% (6/6) of type II capillary pattern. The only two lesions with type V capillary pattern and type Vi pit pattern were LST-NG-PD, and all other lesions of this subtype showed type III capillary pattern (p = 0.04) and type III pit pattern. All six lesions with type II capillary pattern were sessile serrated adenomas, and five of them (83.3%) were located in the right colon.
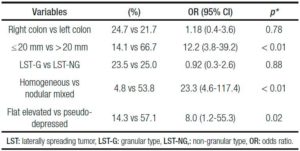
Twenty-three (24%) lesions had advanced histology in 21 patients (14 female, 66.7%). Advanced histology was significantly more common among lesions > 20 mm (p < 0.001) and in lesions with capillary pattern suggestive of neoplasms (0/6 for type II pattern, 9/62 [14.5%] for type III pattern, 12/26 [46.1%] for type IV pattern, and 2/2 [100%] for type V pattern; p = 0.001). Lesions with advanced histology were larger than all other lesions (25.1 ± 10 mm vs 14.3 ± 5 1 mm; p < 0.01). There was no difference between the right colon and left colon (24.7% vs 21.7%, p = 1.0) or between LST-G and LST-NG, but a difference was observed between their respective subtypes (homogeneous [4.8%] vs nodular mixed [53.9%], p < 0.001; and flat elevated [14.3%] vs pseudodepressed [57.1%], p = 0.04).
Logistic regression analysis showed that LSTs > 20 mm, LST-NG-PD, and LST-G-N were predictors of advanced histology (Table 4).
Table 4. Logistic regression analysis of advanced histology.
Seventy-eight patients with 78 LSTs had follow-up examination at 6, 12, and 18 months after EMR. There were five recurrences (6.4%). Recurrences were more common among lesions > 20 mm treated with piecemeal EMR than in lesions ≤ 20 mm (28.6% vs 1.6%, p = 0.003) and in lesions with advanced histology (22.2% vs 1.7%, p = 0.009). The mean size of recurrent lesions was 36 ± 11.9 mm (p < 0.01). The LST-G-N subtype was associated with more recurrences (p = 0.05), and showed a larger mean size than all other subtypes (22.3 ± 8.1 mm, p < 0.01).
All five recurrences/residual neoplasms were detected in the first follow-up colonoscopy. Four of them were adenomas and were easily treated with a second EMR, with complete removal of the lesion and no complications. Incomplete resection was found in only one case with massive sub mucosal invasion, requiring additional surgical intervention. No perforations were observed.
Discussion
LSTs are recognized as precursors of colorectal cancer. Prevalence of LSTs, however, is not high. A large multicenter study21 reported a prevalence of 0.93% among all colonoscopies and of 4.5% among patients with precancerous lesions, with a male predominance (55%) and a mean patient age of 66.2 years. Kim et al showed a prevalence of 2.35% among 6499 patients with colorectal adenomas, also reporting a male predominance, with a mean patient age of 62.5 years.22 Recently it was observed a prevalence of LSTs five times higher in the USA than in South Korea (2.6% vs 0.5%, p < 0.001).23 Most studies on LSTs are conducted in Asia, and there is little published information on these lesions outside of Asia, especially in Latin America, which has prompted this research. Our overall prevalence was 2.5% in 3.623 consecutive colonoscopies and 8% among patients with premalignant lesions, accounting for 4.6% of superficial neoplastic lesions and 10% of non-polypoid neoplasms. LSTs were more commonly found in women (69.2%), and the mean patient age was 67 years.
Most LSTs reported in the literature are of the granular type and predominant in the right colon, which is consistent with our findings of 70.8% and 76%, respectively.21, 22, 24 There was no difference in size between LST-G and LST-NG in ourseries. However, in line with results of previous studies, LST-G-Ns were significantly larger than all other subtypes.24, 25 Most LST-G-Hs and LST-NG-FEs were adenomas with low-grade dysplasia (95.2% and 85.7%, respectively) and were located in the right colon (76.2% and 66.7%, respectively). LST-NG-PDs were also predominant in the right colon (71.4%).
Digital chromoendoscopy (using Flexible Intelligent Color Enhancement, FICE) or real-time chromoendoscopy (using indigo carmine) allowed us to determine lesion morphology and define their pit and capillary patterns, influencing the choice of approach to LST management. Similar to the study by Huang et al, in which type IV was the main pit pattern in the LST-G type, we also found a higher frequency of type IV pit pattern in LST-Gs, especially in the LST-G-N subtype.10 In our series, 90 lesions showed type III-V capillary patterns, which are suggestive of neoplastic lesions, and six lesions showed type II capillary pattern (all of them were LST-NG-FE), which is suggestive of non-neoplastic lesion. Five of them were located in the right colon, and one in the rectum. All six lesions were removed, following the recommendations of Rex et al, and histopathological analysis revealed that they were sessile serrated adenomas.6
Two lesions of the LST-NG-PD subtype showed type V capillary pattern and type Vi pit pattern along with advanced histology. Advanced histology (adenoma with high-grade dysplasia or early cancer) was observed in 21 LSTs (23%), and was more commonly detected in lesions > 20 mm (p < 0.001), in capillary patterns suggestive of neoplasms (III-V; p = 0.001), in LST-G-N (p < 0.001), and in LST-NG-PD (p = 0.043). Previous studies have identified large lesions, with pseudodepression and large nodules (≥ 10 mm), as predictors of higher aggressiveness of LSTs.22, 24, 25 Unlike most studies, Xu et al26 found no difference between LST-NG-FE and LST-NG-PD subtypes. Non-polypoid lesions tend to be more aggressive than polypoid lesions, with an increased risk of advanced histology.27
Most LSTs are adenomatous lesions, even when they reach large diameters, allowing endoscopic resection to be performed. Prior to selection of the resection technique, it is important to distinguish between adenoma and adenocarcinoma. According to the guidelines of the Japan Gastroenterological Endoscopy Society (JGES),28 using magnifying endoscopy, known as optical biopsy, is more effective and highly accurate both for distinction between adenoma and adenocarcinoma and for evaluation of invasion depth than carrying out a simple biopsy, which, in addition to not providing a qualitative diagnosis, may cause fibrosis, thus hindering endoscopic treatment.
En bloc resection is the optimal treatment of colorectal neoplasms, especially adenocarcinomas, facilitating histopathological diagnosis and allowing lymphovascular invasion and invasion depth to be properly evaluated. Lesions up to 20 mm in size can be easily removed by EMR. The choice between piecemeal EMR and endoscopic submucosal dissection (ESD) for large LSTs should be based on LST subtype with magnification. LST-G-N with large nodule and LST-NG-PD, which may have multifocal submucosal invasion, whose foci are often difficult to detect, should be removed preferably by en bloc resection, for which ESD is indicated, with reduced recurrence rates.9 Colorectal ESD is also indicated for lesions > 20 mm, lesions that are difficult to be removed en bloc by EMR, lesions with type Vi pit pattern, carcinomas with shallow submucosal invasion, mucosal tumors with fibrosis, and recurrent or residual early carcinomas after endoscopic resection.9, 28 In our series, all seven cases of LST-NG-PD were ≤ 20 mm, and were removed by en bloc EMR. The ESD technique, however, is more complex than EMR and has been associated with a long learning curve and more complications, especially perforation, and its use should be limited to specialized centers.26
For early detection of local recurrence, periodic observation with colonoscopy is required. Oka et al29 showed that piecemeal resection, LST-G ≥ 40 mm, no pre-treatment magnification, and ≤ 10 years of experience in endoscopic resection were significant risk factors for local recurrence. Recurrence has also been associated with resection of more pieces of LST.30 In our series, 78 patients with 78 LSTs underwent follow-up colonoscopy at 6, 12, and 18 months after EMR. Five patients (6.4%) showed evidence of recurrence/residual neoplasm, and all cases were detected in the first follow-up examination. Recurrences were more common in lesions > 20 mm treated with piecemeal EMR and lesions with advanced histology, and LST-G-N was the subtype with the highest recurrence rate.
Even with a rate of about 28% (4/18) of recurrence/ residual neoplasm for lesions removed by piecemeal EMR in our series, only one case required additional surgical intervention, because it was an LST-G-N 50 mm in size located in the right colon, which showed massive submucosal invasion on histopathological analysis. Therefore, piecemeal EMR is an acceptable option, and is still an effective option for resection of large mucosal tumors.31, 32
Our study has some limitations. It was conducted in one endoscopy center and all colonoscopies were performed by a single endoscopist, who had extensive experience in magnification and colorectal resection by EMR, but without expertise in ESD.
In conclusion, the prevalence of LSTs is small and most LSTs are adenomas that can be treated endoscopically. Selection of the resection technique should be based on lesion size and morphological subtype, and magnifying endoscopic observation of lesions may be useful for evaluation of their features. LSTs > 20 mm and pseudodepressed and nodular mixed subtypes were considered risk factors for advanced histology. EMR, even using the piecemeal technique, is a good option for the treatment of LSTs. Early control in patients with lesions larger than 20 mm, which were resected piecemeal (picemeal technique), especially pseudodepressed and nodular mixed subtypes should be performed.
Referencias
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687-696.
- Morson BC. Evolution of cancer of the colon and rectum. Cancer 1974; 34: 845-849.
- Kariya A. A case of early colonic cancer type IIc associated with familial polyposis coli. I to Cho (Stomach and Intestine) 1977; 12: 1359-1364.
- Muto T, Kamiya J, Sawada T et al. Small » flat adenoma» of the large bowel with special reference to its clinicopathologic features. Dis Colon Rectum 1985; 28: 847-851.
- Jass JR, Baker K, Zlobec I,Higuchi T, Barker M, Buchanan D, Young J. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a ‘fusion’ pathway to colorectal cancer. Histopathology 2006; 49: 121-124.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, O’Brien MJ, Odze RD, Ogino S, Parry S, Snover DC, Torlakovic EE, Wise PE, Young J, Church J. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol2012; 107: 1315-1329.
- Ishigooka S, Nomoto M, Obinata N, Oishi Y, Sato Y, Nakatsu S, Suzuki M, Ikeda Y, Maehata T, Kimura T, Watanabe Y, Nakajima T, Yamano HO, Yasuda H, Itoh F. Evaluation of magnifying colonoscopy in the diagnosis of serrated polyps. World J Gastroenterol 2012; 18: 4308-4316.
- Kudo S, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T, Tanaka S, Watanabe H, Sung JJ, Feld AD, Inadomi JM, O’Brien MJ, Lieberman DA, Ransohoff DF, Soetikno RM, Triadafilopoulos G, Zauber A, Teixeira CR, Rey JF, Jaramillo E, Rubio CA, Van Gossum A, Jung M, Vieth M, Jass JR, Hurlstone PD. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc 2008; 68: S3-S47.
- Tanaka S, Saitoh Y, Matsuda T, Igarashi M, Matsumoto T, Iwao Y, Suzuki Y, Nishida H, Watanabe T, Sugai T, Sugihara K, Tsuruta O, Hirata I, Hiwatashi N, Saito H, Watanabe M, Sugano K, Shimosegawa T. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol 2015; 50: 252-260.
- Huang Y, Liu S, Gong W, Zhi F, Pan D, Jiang B. Clinicopathologic features and endoscopic mucosal resection of laterally spreading tumors: experience from China. Int J Colorectal Dis 2009; 24: 1441-1450.
- Santos CE, Pereira-Lima JC, Lopes CV, Malaman D, Parada AA, Salomão AD. Comparative study between MBI (FICE) and magnification chromoendoscopy with indigo carmine in the differential diagnosis of neoplastic and non-neoplastic lesions of the colorectum. Arq Gastroenterol 2009; 46: 111-115.
- dos Santos CE, Lima JC, Lopes CV, Malaman D, Salomão AD, Garcia AC, Teixeira CR: Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol 2010; 22: 1364-1371.
- Dos Santos CE, Malaman D, Lopes CV, Pereira-Lima JC, Parada AA. Digital chromoendoscopy for diagnosis of diminutive colorectal lesions.Diagn Ther Endosc 2012; 2012: 279521.
- Oliveira dos Santos, CE; Perez HJ; Mönkemüller K; Malaman D; Lopes CV; Pereira-Lima JC. Observer agreement for diagnosis of colorectal lesions with analysis of the vascular pattern by image-enhanced endoscopy. Endoscopy International Open 2015; 3: E240-E245.
- Kanao H, Tanaka S, Oka S, Hirata M, Yoshida S, Chayama K: Narrow-band imaging magnification predicts the histology and invasion depth of colorectal tumors. Gastrointest Endosc 2009; 69: 631-639.
- Santos CE, Malaman D, Pereira-Lima JC: Endoscopic mucosal resection in colorectal lesion: a safe and effective procedure even in lesions larger than 2 cm and in carcinomas. Arq Gastroenterol 2011; 48: 242-247.
- Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A: Colorectal tumours and pit pattern. J Clin Pathol 1994; 47: 880-885.
- Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H: Diagnosis of colorectal tumours lesions by magnifying endoscopy. Gastrointest Endosc 1996; 44: 8-14.
- Teixeira CR, Torresini RS, Canali C, Figueiredo LF, Mucenic M, Pereira Lima JC, Carballo MT, Saul C, Toneloto EB: Endoscopic classification of the capillary-vessel pattern of colorectal lesions by spectral estimation technology and magnifying imaging. Gastrointest Endosc 2009; 69: 750-756.
- The Paris endoscopic classification of superficial neoplastic lesions: Gastrointest Endosc 2003; 58: S3-S43.
- Rotondano G, Bianco MA, Buffoli F, Gizzi G, Tessari F, Cipolletta L. The Cooperative Italian FLIN Study Group: prevalence and clinico-pathological features of colorectal laterally spreading tumors. Endoscopy 2011; 43: 856-861.
- Kim BC, Chang HJ, Han KS, Sohn DK, Hong CW, Park JW, Park SC, Choi HS, Oh JH. Clinicopathological differences of laterally spreading tumors of the colorectum according to gross appearance. Endoscopy 2011; 43: 100-107.
- Cha JM, Kozarek RA, La Selva D, Gluck M, Ross A, Chiorean M, Koch J, Lin OS. Disparities in prevalence, location, and shape characteristics of colorectal neoplasia between South Korean and U.S. patients. Gastrointest Endosc 2015; 82: 1080-1086.
- Kaku E, Oda Y, Murakami Y, Goto H, Tanaka T, Hasuda K, Yasunaga M, Ito K, Sakurai K, Fujimori T, Hattori M, Sasaki Y. Proportion of flat- and depressed-type and laterally spreading tumor among advanced colorectal neoplasia. Clin Gastroenterol Hepatol 2011; 9: 503-508.
- Kim KO, Jang BI, Jang WJ, Lee SH. Laterally spreading tumors of the colorectum: clinicopathologic features and malignant potential by macroscopic morphology. Int J Colorectal Dis 2013; 28: 1661-1666.
- Xu MD, Wang XY, Li QL, Zhou PH, Zhang YQ, Zhong YS, Chen WF, Ma LL, Qin WZ, Hu JW, Yao LQ. Colorectal lateral spreading tumor subtypes: clinicopathology and outcome of endoscopic submucosal dissection. Int J Colorectal Dis 2013; 28: 63-72.
- dos Santos CE, Malaman D, Mönkemüller K, Dos Santos Carvalho T, Lopes CV, Pereira-Lima JC. Prevalence of non-polypoid colorectal neoplasms in southern Brazil. Dig Endosc 2015; 27: 361-367.
- Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo SE, Tsuruta O, Sugihara K, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H. JGES guidelines for colorectal endoscopic submucosal dissection/ endoscopic mucosal resection. Dig Endosc 2015; 27: 417-434.
- Oka S, Tanaka S, Saito Y, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisabe T, Tsuruta O, Sano Y, Yamano H, Shimizu S, Yahagi N, Watanabe T, Nakamura H, Fujii T, Ishikawa H, Sugihara K. Local Recurrence After Endoscopic Resection for Large Colorectal Neoplasia: A Multicenter Prospective Study in Japan.Am J Gastroenterol 2015; 110: 697-707.
- Terasaki M, Tanaka S, Oka S, Nakadoi K, Takata S, Kanao H, Yoshida S, Chayama K. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol 2012; 27: 734-740
- Chang DK. Many options to manage laterally spreading tumors. ClinEndosc 2015; 48: 4-5.
- Santos CE, Malaman D, Carvalho Tdos S, Lopes CV, Pereira-Lima JC. Malignancy in large colorectal lesions. Arq Gastroenterol 2014; 51: 235-239.
Correspondencia: Carlos Eduardo Oliveira dos Santos
Rua Gomes Carneiro, 1343
CEP 96400-130, Bagé, RS, Brasil
Tel/fax: 55-53-32410808
Correo electrónico: ddendo@uol.com.br
Acta Gastroenterol Latinoam 2016;46(3): 184-191
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE