Manuel Valero,1 Ana María Bosco,2 Claudia Milano1
1 Department of Gastroenterology.
2 Department of Endoscopy, Hospital Nacional Prof. Alejandro Posadas, University of Buenos Aires (UBA). Pte. Illia s/n, 1684. El Palomar.
Acta Gastroenterol Latinoam 2017;47: 29-37
Recibido: 09/01/2016 / Aprobado: 17/10/2016 / Publicado en www.actagastro.org el 05/04/2017
Summary
Aims. To evaluate if the co-administration of itopride to the 4 liters of polyethylene glycol preparation (PEG 4 L), improves patient tolerance and acceptability, and increases the colonic cleansing quality. Material and methods. It was a prospective, randomized, double blind study. The patients were randomized into two groups (PEG 4 L + placebo and PEG 4 L + itopride 100 mg). A questionnaire with demographic, socio-cultural and satisfaction data was completed and the effectiveness of bowel cleansing was measured according to the Boston Bowel Preparation Scale (BBPS). Results. A total of 53 patients, median age 56 years (range: 78-18), 64% women, were distributed in two groups (24 in itopride group and 29 in control group). The preparation was easier to perform using the prokinetic (58.3% vs 41.4%; p = 0.086) and also the taste was considered more pleasant and tolerable. While there was a trend to improve all undesirable effects, a significant difference was found only with the upper gastrointestinal symptoms (nausea + vomiting). All of the patients in the itopride group would repeat the colonoscopy if necessary (100% vs 79.3%; p = 0.018). A better colonic preparation was observed, as a result of improving the right colon cleansing (p = 0.012), and the procedure time was shortened (23 min vs 18 min; p = 0.026). However, there was not an increment in the lesion detection rate. Conclusions. The co-administration of itopride together with PEG solution improved patient tolerance and acceptability. Although there was an increment in bowel cleansing quality, itopride was not superior to the placebo in the lesion detection rate. It is necessary to be cautious in interpreting some of the results until more evidence is available in the literature.
Key words. Itopride, prokinetic, bowel preparation, colonoscopy.
Eficacia del itopride en la preparación para la colonoscopía: un estudio prospectivo aleatorizado doble ciego
Resumen
Objetivo. Evaluar si la administración conjunta de itopride a la preparación de 4 litros de polietilenglicol (PEG 4 L), mejora la tolerancia y la aceptabilidad del paciente, y aumenta la calidad de la limpieza del colon. Material y métodos. Estudio prospectivo, aleatorizado, doble ciego. Los pacientes fueron distribuidos aleatoriamente en dos grupos (PEG 4 L + placebo y PEG 4 L + itopride 100 mg). Se completó un cuestionario con datos demográficos, socio-culturales y de satisfacción. Se evaluó la eficacia de la limpieza intestinal de acuerdo a la Boston Bowel Preparation Scale (BBPS). Resultados. Un total de 53 pacientes, edad mediana: 56 años (rango: 78-18), 64% mujeres, fueron distribuidos en dos grupos (24 en el grupo itopride y 29 en el grupo control). La preparación fue más fácil de realizar utilizando el procinético (58,3% vs 41,4%; p = 0,086), así como el sabor más agradable y tolerable. Si bien hubo una tendencia a mejorar todos los efectos indeseables, se encontró una diferencia significativa sólo en los síntomas gastrointestinales altos (náusea + vómitos). Todos los pacientes con itopride repetirían la colonoscopía de ser necesario (100% vs 79,3%; p = 0,018). Se logró una mejor preparación colónica, a expensas de una mayor limpieza del colon derecho (p = 0,012) y se acortó el tiempo de duración de la colonoscopía (23 min vs 18 min; p = 0,026). Sin embargo, no hubo una mejora en la tasa de detección de lesiones. Conclusiones. La administración conjunta de itopride y PEG, mejoró la tolerancia y la aceptabilidad del paciente. Si bien, se logró mejorar la calidad de la limpieza intestinal, el itopride no fue superior al placebo en la tasa de detección de lesiones. Es necesario ser cautos en la interpretación de los resultados hasta que se disponga de más evidencia en la literatura.
Palabras claves. Itopride, procinéticos, preparación intestinal, colonoscopia.
Abbreviations
PEG: polyethylene glycol.
SF: sodium phosphate.
BBPS: Boston bowel preparation scale.
CRC: colorectal cancer.
BMI: body mass index.
Colorectal cancer (CRC) is the most frequent gastrointestinal tumor and the second cause of cancer related death.1 Although the colonoscopy is the recommended method for CRC prevention, requires optimal visualization of the intestinal mucosa.2
Two essential indicators of colonoscopy quality are the cecal intubation rate and the detection rate of polyps. Both depend on bowel cleansing.3, 4 It is estimated that about 20% of colonoscopies have an inadequate preparation.5 This is associated with lengthy procedures and less detection of adenomas, reduces the screening intervals and increases the costs and risks of complications. This causes frustration for the patient and physician with medico-legal conflicts.4, 6
The ideal cleansing method must be safe, well tolerated and effective; however, none of the current options fulfills these characteristics. Several oral solutions have been evaluated but only polyethylene glycol (PEG) and sodium phosphate (SF) have shown the best results. Both are equally effective and can be administrated alone or in combination with other drugs aiming to improve the quality of the preparation and patient tolerability.7 PEG has the advantage of being safer. It does not have the risk of severe electrolyte disturbances and renal failure that SF has, however it is difficult to ingest large volumes (4 L) of an unpleasant tasting solution and may develop symptoms of intolerance (5-15% of the patients). The main cause of inappropriate cleansing (80% of cases), is a failure to adequately follow preparation instructions and mostly because of intolerance to the oral solution.8, 9
The aim of the present study was to evaluate if the co-administration of itopride to PEG 4 L preparation, improved the tolerance to the oral solution and patient satisfaction, with the reduction of undesirable effects. Simultaneously, it was analyzed if there was an improvement of bowel cleansing, and colonoscopy quality determined by the cecal intubation and lesion detection rates.
Material and methods
Study Design
It was a prospective, randomized, double blind, placebo-controlled study, conducted by the Endoscopy and Gastroenterology Units of the Hospital Profesor A. Posadas, between September and December of 2014. The study protocol and consent form were approved by the Institutional Review board and was conducted according to the Helsinki declaration. After the participants signed an informed consent, were randomized using a system of sealed opaque envelopes.
Patient selection
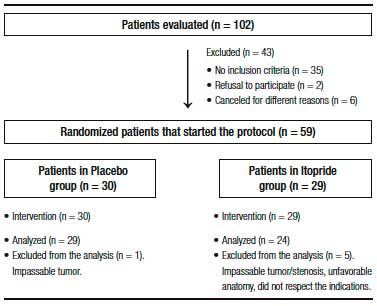
Participants included were outpatients with a clear indication for colonoscopy. Exclusion criteria were age under 18 or over 80 years old, hospitalized patients, pregnancy or lactation, allergy to itopride or benzamides, severe illness (heart, renal or liver failure) or metabolic disease, Parkinson’s disease, ascites, suspected colonic obstruction or history of previous obstruction, gastrointestinal bleeding, history of small bowel resection or colorectal surgery, patients with an ileostomy/colostomy, history of gastrectomy, gastric bypass or feeding gastrostomy, patients with difficulty understanding instructions or refusal to consent to participate in the study. Patients who did not carry out the preparation instructions, as well as those, whose colonoscopy had to be aborted because it was not possible to reach the cecum due to an unfavorable anatomy or a benign/malignant stenosis, were excluded from the statistical analysis (Figure 1).
Group description
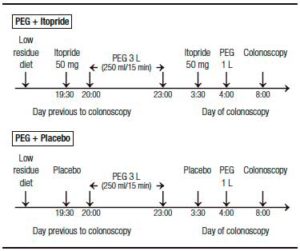
Patients were randomized in two groups. One group received PEG + a manufactured glucose placebo and the other PEG + itopride (itopride hydrochloride – AFLUSAN 50 mg tablets, Dominguez Laboratory, Argentina and DAGLA 50 mg tablets, Takeda Pharmaceuticals, U.S.A). Both, patients and endoscopists were blind to the group selection. PEG administration was performed following current literature recommendations. The split-dose preparation was chosen in order to reduce side effects such as nausea, vomiting and abdominal distention.10 In both groups, 3 liters (L) of PEG were administered the day before to the procedure at 8:00 pm and 1 L the day of the colonoscopy at 4:00 am. All of the participants received two tablets in a closed envelope (2 tablets of itopride 50 mg or 2 tablets of placebo) and each tablet had to be ingested 30 minutes before the PEG dose (Figure 2). The total itopride dose used (2 tablets of 50 mg) was designated empirically and corresponds to the recommended dose for functional dyspepsia treatment (main current indication of the itopride).11
Figure 1. Flow chart of patients.
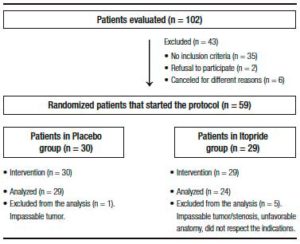
Figure 2. Colonoscopy preparation. PEG: polyethylene glycol.
The colonoscopies were performed in the morning (from 8:00 to 12:00 am), in order to prevent an interval of more than 4 hours between the last ingestion of the solution and the procedure, under propofol sedation and by 4 endoscopists with experience using the BBPS, in order to reduce an operator bias.12 Only the procedures in which the cecum was reached were analyzed. Both groups underwent the same diet the day before (low residue diet for breakfast and liquid diet starting from lunch).13, 14
Table 1. Demographic and socio-cultural data.
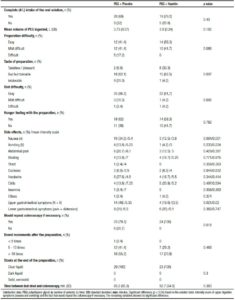
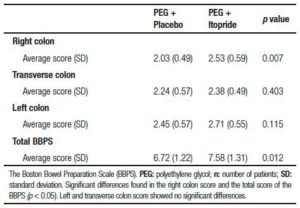
The evaluation of patient tolerance and acceptability was conducted using a questionnaire that patients were required to complete before the colonoscopy, including demographic, constitutional, socio-cultural and satisfaction data (Table 1-3). The effectiveness of the bowel cleansing was measured using the Boston Bowel Preparation Scale (BBPS) which allows a segmental evaluation (right colon, transverse and left colon), using a four grade scale (0: Solid stool that cannot be cleared, 1: Residual stool and/or opaque liquid that prevents the complete evaluation of the mucosa, 2: Minor amount of opaque liquid or clear liquid that allows the evaluation of the entire mucosa, 3: Absence of any residue with full eval uation of the mucosa).15 Thereby, each segment of the colon was scored and the final score was calculated. The total score ranged from 0 to 9 with the highest score for better preparation. Data from the colonoscopy results and the BBPS are detailed in Tables 4 and 5.
Table 2. Patient constitutional data.
Statistical Analysis
The statistical interpretation of the results was performed, blindly, by the Department of Education and Research. For sample size calculation, two numerical variables were taken into account: a) Volume of PEG ingested; b) BBPS scored, with the intention to reach a mean difference between groups of at least 500 ml of ingested PEG and two points at the BBPS. With an alpha error of 0.05 and a power value of 0.9, the sample size selected was 100 patients. However, given that the use of itopride is not a formal indication for bowel preparation, the lack of previous well-designed studies evaluating the safety of this drug as an adjunct to PEG, and the lack of knowledge of the effective dose required, the Institutional Review Board determinated the need to perform a partial evaluation of success and side effects after reaching 50 patients. At that time, after the analysis, it was found that the expected benefit was reached in almost every variable, so an assessment of risk-benefit analysis was carried out, taking into account the reasons already stated, and it was decided by internal consensus to end the study. Chi-square or Fisher’s exact test were used to evaluate categorical variables and the Mann-Whitney test for continuous variables. All statistical analysis was performed using the SPSS software suite v.22.
Results
A total of 59 patients (PEG + itopride: 29 and PEG + placebo: 30) consented to participate in the protocol. However, 6 patients were subsequently excluded from the analysis because of different reasons (Figure 1) and finally a total of 53 patients, 24 in the itopride group and 29 in the control group were analyzed.
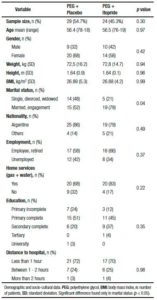
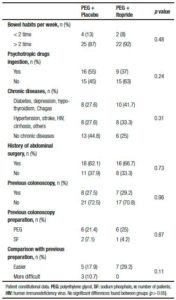
Comparison of demographic and socio-cultural data is shown in Table 1. There was no difference between groups in terms of sample size, age, gender and body mass index (BMI). The average age was 56 years old, 19 (36%) men and 34 (64%) women. The mean BMI was of 26.8 kg/m2. Socio-cultural variables were included in order to avoid any bias. The marital status, nationality, occupation, housing type, education, and distance to the hospital are factors that could influence directly or indirectly colon cleansing. All these variables except marital status were equally distributed between the two groups, but iy was a condition to be included in the protocol to have someone that would help with the oral solution if needed, so the difference in marital status was not substantial.
Table 5. The Boston Bowel Preparation Scale (BBPS).
The constitutional data showed no differences between groups in regard to the bowel habits of the participants, the ingestion of psychotropic drugs, chronic illnesses or surgeries. A history of previous colonoscopies was considered, anticipating that a bad experience with the oral solution, could lead to the failure of further procedures. Most of the participants did not have a previous colonoscopy, and among those who did have, the 80% used PEG solution for colonic cleansing and considered the new preparation with itopride more tolerable (29.2% vs 17.9%; p > 0.05) (Table 2).
Satisfaction data is shown in Table 3. Although both groups consumed almost the same mean volume of PEG (placebo: 3.73 Lvs. itopride: 3.9 L; p > 0.05), most patients using the prokinetic ingested the full 4-liters solution (79.2% vs 68%; p > 0.05), considered the preparation easier to perform (58.3% vs 41.4%; p = 0.086) and the flavor more pleasant and tolerable (p = 0.007). There was no difference between groups in the difficulty of performing the previous day`s diet or the hungry feeling with the preparation (p > 0.05).
The development of side effects resulting from the solution intake and its intensity were analyzed. With regard to side effects frequency, a significant benefit was seen only in upper digestive tract symptoms (nausea and vomiting). In case of, mild abdominal pain (most common side effect of this prokinetic) and the others undesirable effects, despite there was a trend towards improvement, the difference between the groups was negligible (p > 0.05). A visual analog scale with values from 0 to 10 (0: no symptom and 10: worst possible symptom) was used to measure side effects intensity and showed that, although there was a clear benefit with the itopride use only with nausea and vomiting symptoms (p = 0.02), a lower score was observed concerning most side effects. Also all the participants in the prokinetic group would repeat the colonoscopy if necessary (100% vs 79.3%; p = 0.018) and a higher number of bowel movements were achieved (70.8% >10 times; p = 0.469).
The colonoscopy data is summarized in Table 4. The most common colonoscopy indication in both groups was the CRC screening. The mean procedure time was shortened in the prokinetic group (23 min vs 18 min; p = 0.026) as a result of improving the withdrawal time (p = 0.039). In both groups the withdrawal time of 6 minutes currently recommended was respected.16 No differences were found regarding to the polyp and adenoma detec tion rates. In both groups, the rates were higher than 20% and met the average standards for colonoscopy screening.16 The number of polyps detected per patient and the mean size of them was not different between the groups (p > 0.05). In the control group one case of cancer was found and more biopsies were performed (p = 0.007).
The bowel cleansing effectiveness was measured using the Boston Bowel Preparation Scale (Table 5). The average score was calculated in each segment of the colon with dispersion parameters. There was no improvement in the left and transverse colon cleansing using the prokinetic, but the right colon showed a significant benefit (p = 0.007), allowing a higher total score (p = 0.012).
Discussion
A clean colon is essential for the diagnostic accuracy and therapeutic safety of colonoscopy. The introduction of PEG has improved the quality of bowel preparation. However, the need to ingest a large volume of solution (4 L), the unpleasant taste and the undesirable side effects, are often the reasons for preparation failure.
Multiple prokinetics have been tested in order to improve the tolerability and effectiveness of PEG. Domperidone, metoclopramide and tegaserod did not improved patient tolerance or colon cleansing.17 Cisapride has shortened the precolonoscopic period of preparation and decreased the volume needed of the solution.18 However, these results have been difficult to reproduce and also serious side effects have been reported.19 Domperidone and metoclopramide may cause extra pyramidal symptoms with long-term use.20 Cisapride was withdrawn from the market due to severe cardiac side effects, including QT prolongation and ventricular arrhythmias.21 Some international trials have used the mosapride and itopride in combination with PEG. Mishima et al. improved, with the co-administration of mosapride and itopride, patient tolerance and acceptability, but without a substantial increment in the bowel cleansing.22 Other studies showed that, using mosapride at doses of 15 mg, could improve tolerance and colon cleansing, especially in patients with severe constipation.23, 24
Itopride is a prokinetic derived from benzamide, an antagonist of dopamine D2 receptors and an inhibitor of acetylcholinesterase. It is commonly used to treat functional dyspepsia, nausea and vomiting, delayed gastric emptying, anorexy and regurgitation. It acts through the increment of gastrointestinal peristalsis. Although, it mainly stimulates gastric motility and accelerates gastric emptying, it has a prokinetic effect in the entired gastrointestinal tract and improves colonic peristalsis and stool propulsion.20, 25, 26 These facts explain why it can relieve PEG related discomfort and improves colonic cleansing. Some authors have reported that the combination of itopride with PEG or SF, can reduce abdominal symptoms but with a subtle improvement in the colon cleansing.22 However, others like Kim HJ et al. showed, in a prospective trial using 200 mg of itopride simultaneously with split-dose of PEG, that the patients using the prokinetic had a significant higher efficacy in bowel cleansing and a less obvious improvement of adverse effects than patients in the split-dose of PEG alone group.27 The fact is that, although several studies using prokinetics have shown successful results, all of them agree that more evidence is necessary for its recommendation.22-24
This study showed that, despite both groups having consumed almost the same amount of PEG, most patients considered the itopride preparation easier to perform and the flavor more pleasant and tolerable. Although, there was a significant benefit only with the upper digestive symptoms (nausea and vomiting), the rest of undesirable effects showed a tendency to improve and all patients in this group would repeat the colonoscopy if necessary. These findings support the efficacy in terms of patient acceptability and tolerance. With regard to the effectiveness of the colon cleansing process, a significant advantage in BBPS total score was seen as a result of a better preparation of the right colon, and the procedure time was shortened.
It is now well accepted that an adequate bowel cleansing led to a higher rate of adenomas detection, but only a few studies were designed to assess bowel preparation quality for colonoscopy and have also examined the lesions detection rate.28-30 This study showed no differences between groups in terms of polyp/adenoma detection rate, size and number of polyps per patient. However, polyp detection depends on several variables, such as inherent factors of the patient, the indication of the colonoscopy, endoscopic technique and the technology available.30 Thus additional studies are needed to conclude about the relationship between bowel preparation using itopride and lesion detection rate.
One limitation of this trial was the sample size. Perhaps future studies with larger populations may clarify the benefits of using this prokinetic and determinate whether itopride definitely can improve the side effects, optimize the quality of bowel cleansing and hence the lesion detection rate. Other limitation was the use of a non-standardized dose. The dose selected aimed at preventing adverse events related to itopride and it is possible that may have been inadequate for achieving a greater benefit. However, Kim HJ and col. used in their trial a higher dose (200 mg) of itopride with similar results.27 Finally, this was a study performed in a single center, which limits the external validity.
Conclusion
The co-administration of itopride together with PEG solution improved tolerance and patient acceptability. Although there was an increment in bowel cleansing quality, itopride was not superior to the placebo in the lesion detection rate. It is necessary to be cautious in interpreting some of the results until more evidence is available in the literature.
Author contributions. Drago Daniel, Department of Education and Research, Hospital Nacional Prof. A. Posadas, guided the design of the protocol and the statistical analysis of the results.
Conflicts of interest. The authors have no financial support or conflict of interest.
Referencias
- US Cancer Statistics Working Group. United States Cancer Statistics: 1999 – 2005 Incidence and Mortality Web-Based Report. US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute: Atlanta, 2009.
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology Guidelines for Colorrectal Cáncer Screening 2008. Am J Gastroenterol 2009; 104: 739-750.
- Centers for Disease Control and Prevention. Vital signs: Colorectal Cancer Screening, Incidence, and Mortality – United States, 2002–2010. MMWR Morb Mortal Wkly Rep 2011; 60: 884-890.
- Harewood GC, Sharma VK. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003; 58: 76-79.
- Ness R, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol 2001; 96: 1797-1802.
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: The European Panel of Appropriateness of Gastrointestinal Endoscopy European Multicenter Study. Gastrointest Endosc 2005; 61: 378-384
- Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE. A consensus document on bowel preparation before colonoscopy: Prepared by A Task Force from American Society of Colon And Rectal Surgeons, The American Society For Gastrointestinal Endoscopy And The Society Of American Gastrointestinal And Endoscopic Surgeons. Dis Colon Rectum 2006; 49: 792-809.
- Jansen SV, Goedhard JG, Winkens B, van Deursen CT. Preparation before colonoscopy: a randomized controlled trial comparing different regimes. Eur J Gastoenterol Hepatol 2011; 23: 897-902.
- Juluri R, Eckert G, Imperiale T. Polyethylene glycol vs. sodium phosphate for bowel preparation: a treatment arm meta-analysis of randomized controlled trials. BMC Gastroenterology 2011; 11: 38.
- Park JS, Sohn CI, Hwang SJ, Choi HS, Park JH, Kim HJ, Park DI, Cho YK, Jeon WK, Kim BI. Quality and effect of single dose versus split dose of polyethylene glycol bowel preparation for early-morning colonoscopy. Endoscopy 2007; 39: 616-619.
- Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C: A placebo-controlled trial of itopride in functional dyspepsia. N Engl J Med 2006; 354: 832-840.
- Siddiqui AA, Yang K, Spechler SJ, Cryer B, Davila R, Cipher D, Harford WV. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc 2009; 69: 700-706.
- Aoun E, Abdul-Baki H, Azar C, Mourad F, Barada K, Berro Z, Tarchichi M, Sharara AI. A randomized single-blind trial of split-dose PEG-electrolyte solution without dietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc 2005; 62: 213-218.
- Hassan C, Bretthauer M, Kaminski MF, Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green S, Kuiper T, Marmo R, Omar M,Petruzziello L, Spada C, Zullo A, Dumonceau JM. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2013; 45: 142-150.
- Lai E, Calderwood A, Doros G. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009; 69: 620-625.
- Rex DK, Petrini JL, Baron TH. Quality indicators for colonoscopy. Gastrointestinal Endoscopy 2006; 63: 516-528.
- Brady CE, DiPalma JA, Pierson WP. Golytely lavage is metoclopramide necessary? Am J Gastroenterol 1985; 80: 180-184.
- Katsinelos P, Pilpilidis I, Paroutoglou G, Xiarchos P, Tsolkas P, Papagiannis A, Giouleme O, Kapelidis P, Papageorgiou A, Dimiropoulos S, Eugenidis N. The administration of cisapride as an adjuvant to PEG-electrolyte solution for colonic cleansing: a double-blind randomized study. Hepatogastroenterology 2005; 52: 441-443.
- Martínek J, Hess J, Delarive J. Cisapride does not improve precolonoscopy bowel preparation with either sodium phosphate or polyethylene glycol electrolyte lavage. Gastrointest Endosc 2001; 54: 180-185.
- Lim HC, Kim YG, Lim JH, Kim HS, Park H. Effect of itopride hydrochloride on the ileal and colonic motility in guinea pig in vitro. Yonsei Med J 2008; 49: 472-478.
- Tonini M, De Ponti F, Di Nucci A, Crema F. Review article: cardiac adverse effects of gastrointestinal prokinetics. Aliment Pharmacol Ther 1999; 13: 1585-1591.
- Mishima Y, Amano Y, Okita K, Takahashi Y, Moriyama N, Ishimura N, Furuta K, Ishihara S, Adachi K, Kinoshita Y. Efficacy of prokinetic agents in improving bowel preparation for colonoscopy. Digestion 2008; 77: 166-172.
- Tajika M, Niwa Y, Bhatia V, Kawai H, Kondo S, Sawaki A, Mizuno N, Hara K, Hijioka S, Matsumoto K, Kobayashi Y, Saeki A, Akabane A, Komori K, Yamao K. Efficacy of mosapride citrate with polyethylene glycol solution for colonoscopy preparation. World J Gastroenterol 2012; 18: 2517-2525.
- Tajika M, Niwa Y, Bhatia V, Kondo S, Tanaka T, Mizuno N, Hara K, Hijioka S, Imaoka H, Komori K, Yamao K. Can mosapride citrate reduce the volume of lavage solution for colonoscopy reparation? World J Gastroenterol 2013; 7: 727-735.
- Kadowaki M, Wang XO, Shimatani H, Yoneda S, Takaki M. 5-HT4 receptor enhances the propulsive power of peristaltic reflex in the rat distal colon. Auton Neurosci 2002; 99: 62-65.
- Tsubouchi T, Saito T, Mizutani F, Yamauchi T, Iwanaga Y. Stimulatory action of itopride hydrochloride on colonic motor activity in vitro and vivo. J Pharmacol Exp Ther 2003; 306: 787-793.
- Kim HJ, Kim TO, Shin BC, Woo JG, Seo EH, Joo HR, Heo NY, Park J, Park SH, Yang SY, Moon YS, Shin JY, Lee NY. Efficacy of prokinetics with a split-dose of polyethylene glycol in bowel preparation for morning colonoscopy: a randomized controlled trial. Digestion 2012; 86:194-200.
- Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003; 58: 76-79.
- Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: The European Panel of Appropriateness of Gastrointestinal Endoscopy European Multicenter Study. Gastrointest Endosc 2005; 61: 378-384.
- Chiu HM, Lin JT, Wang HP, Lee YC, Wu MS. The impact of colon preparation timing on colonoscopic detection of colorectal neoplasms prospective endoscopist-blinded randomized trial. Am J Gastroenterol 2006; 101: 2719-2725.
Correspondencia: Manuel Valero
Yerbal 896 6C, Buenos Aires. Argentina (CP1405)
Correo electrónico: valeromanu@gmail.com
Acta Gastroenterol Latinoam 2017;47(1): 29-37
 Revista ACTA Órgano Oficial de SAGE
Revista ACTA Órgano Oficial de SAGE